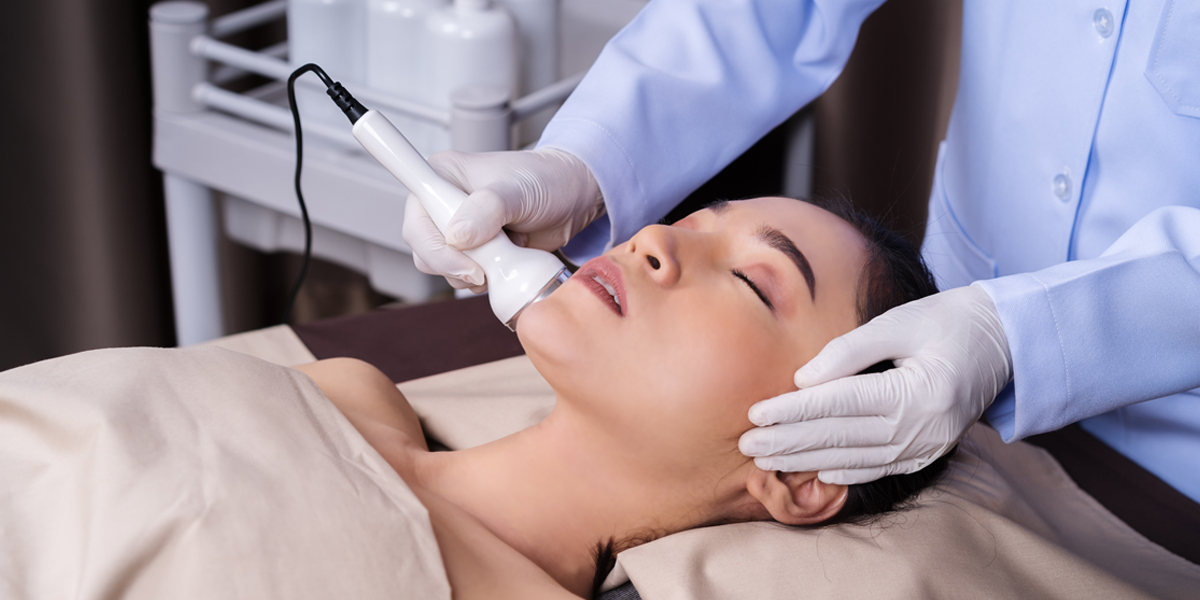
On November 17, 2021, the NMPA (National Medical Products Administration) issued a list of Common Cases of Illegal Use of Beauty Devices and Penalties.
This is a product category currently undergoing close scrutiny by China regulators, so we encourage manufacturers to closely check their compliance status.
After carrying out on-the-spot inspections, the respective AMR (Administration for Market Regulation) officials reported the following violation cases for the use of unregistered medical devices and confiscated the products involved:
Nanjing AMR – January 18, 2021
The medical beauty clinic was found without indicating any Chinese labels of the product, and was fined 98,880 yuan for using an unregistered class III medical device.
Guangzhou Baiyun AMR – April 15, 2021
The beauty equipment company was found without any product information such as the product name, registration certificate, name of manufacturer, etc., displayed at the business location, and was fined 22,100 yuan for using unregistered class II and class III medical devices. In addition, the illegally earned 11,900 yuan profit was confiscated.
Guangzhou Baiyun AMR – March 10, 2021
The medical beauty clinic was found without indicating any Chinese labels of the product, and was fined 89,250 yuan for using unregistered class III medical device.
Meishan AMR – March 16, 2021
The medical beauty company was found without displaying any product information, and was fined 95,700 yuan for using unregistered medical device.
Panzhihua AMR – January 15, 2020
The medical beauty clinic was found without indicating any Chinese labels of the product, and was fined 191,345 yuan for using unregistered medical device. In addition, the illegally earned 40,448 yuan profit was confiscated.
Shanghai AMR – October 12, 2020
The medical beauty clinic in Shanghai was fined 498,000 yuan for using unregistered medical device.
Recently, China authorities have strengthened the supervision and inspection of medical beauty equipment from manufacturers, distributors as well as medical institutions, and have carried out a series of special rectification action to crack down on illegal medical beauty services. Also in June 2021, the National Health Committee (NHC) published (No. 273 – 2021) the Special Work Plan to Combat Illegal Medical Beauty Services to be implemented until December 2021. Contact Cisema if you would like to learn more.
By Alice Liu and Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어