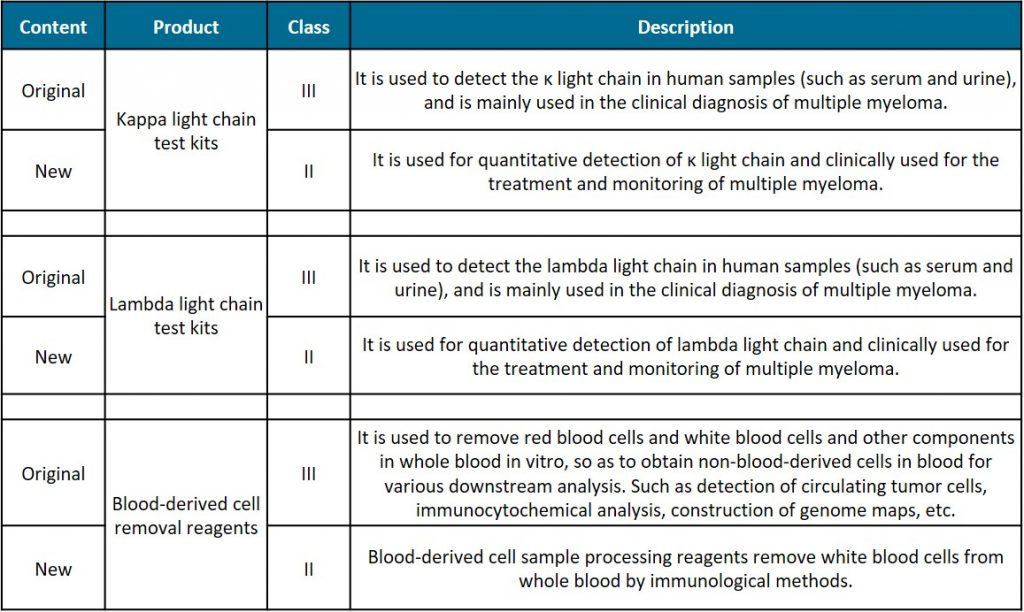
On 24.04.2020, the NMPA (National Medical Products Administration) announced a draft proposal to reclassify some of the IVD reagents in the 6840 Classified Subdirectory (2013 Version), and solicited public opinions until 10.05.2020.
In addition, the draft proposal reclassifies some other IVD reagents including test kits for tumours and cancer diseases.
By Jacky Li. Contact Cisema to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어