The China drug registration evaluation report 2021 has been released by the NMPA’s Center for Drug Evaluation (CDE). We’ve compiled some of the highlights below.
1. Overview of accepted applications
In 2021, CDE had accepted a total of 11,658 registration applications for traditional Chinese medicines, chemical drugs, and biological products, which represents a year-on-year increase of 13.8%. With 47 innovative drugs passing the technical review, setting a record high.
| Registration applications accepted by CDE in 2021 | |||
|---|---|---|---|
| Applications | Number | ||
| Technical review required | Drug Product | 9,231 | 9,235 |
| Drug-device Combination | 4 | ||
| Technical review not required, directly for administrative review | 2,423 | ||
| Total | 11,658 | ||
In 2021, CDE totally approved or recommended the approval of 10,059 drug registration applications.
| Approved or recommended approval by CDE in 2021 | |
|---|---|
| Registration applications category | Approval/recommendation for approval (Number) |
| IND (Investigational New Drug) | 2,108 |
| Validation of clinical trial applications | 59 |
| NDA (New Drug Application) | 323 |
| ANDA (Abbreviated New Drug Application) | 1,003 |
| Consistency evaluation | 1,080 |
| Supplementary application | 2,751 |
| Re-registration application of drugs manufactured abroad | 372 |
| Direct review of registration applications | 2,362 |
| Re-review registration applications | 1 |
| Total | 10,059 |
2. Chemical drug applications requiring technical review
2.1 Accepted chemical drug applications requiring technical review
| Chemical drug application category | Number of applications |
|---|---|
| IND | 1,500 (Including 1,134 IND applications for 487 innovator chemical drugs); |
| Conformity clinical trials | 71 |
| ANDA | 1,791 |
| Quality and therapeutic equivalence evaluations | 908 |
| Supplemental applications | 1,999 |
| License renewals for overseas-manufactured chemical drugs | 322 |
| NDAs | 197 |
| Total | 6,788 |
2.2 Completed chemical drug registration applications requiring technical review
In 2021, CDE has completed 7,295 chemical drug registration applications requiring technical review. In terms of registration application categories, there were:
Fig 1. Number of completed chemical drug registration applications in different types
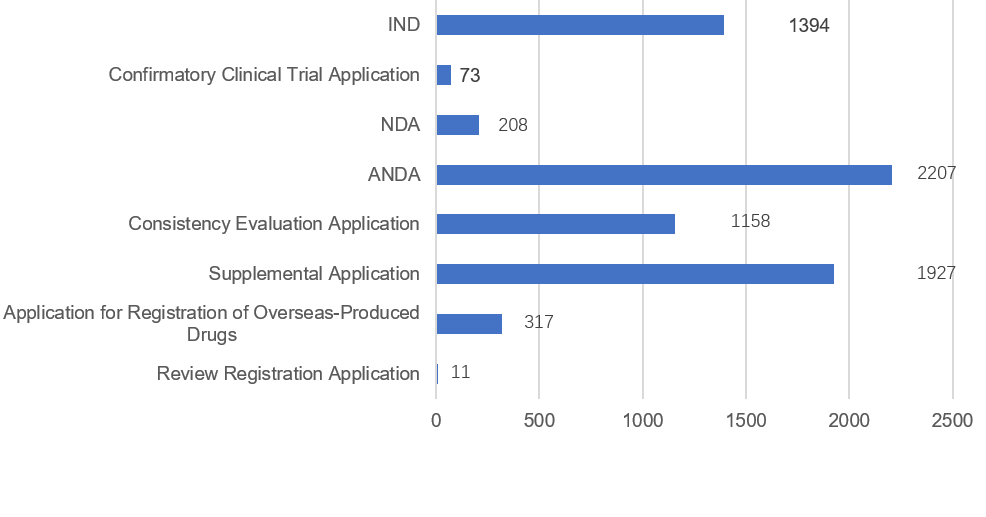
2.3 Approval/recommended approval of chemical drug applications
| Chemical drug applications that needed technical review and were concluded in 2021 | ||||
|---|---|---|---|---|
| Application Type | Approval/Recommended Approval | Disapproval/Recommended Disapproval | Other | Total |
| IND | 1,310 | 26 | 58 | 1,394 |
| Confirmatory Clinical Trial | 59 | 1 | 13 | 73 |
| NDA | 160 | 8 | 40 | 208 |
| ANDA | 1,003 | 394 | 810 | 2,207 |
| Quality and Therapeutic Equivalence Evaluation | 1,080 | 16 | 62 | 1,158 |
| Supplemental Application | 1,673 | 23 | 231 | 1,927 |
| License Renewal Application for Overseas-manufactured Drugs | 305 | 0 | 12 | 317 |
| Re-review | 1 | 7 | 3 | 11 |
| Total | 5,591 | 475 | 1,229 | 7,295 |
| * “Other” refers to the situations that review process were terminated because applicants did not pay the fees according to regulations, withdrew the application, etc. | ||||
Among the 1,394 IND applications for chemical drugs, 1,310 were approved and 26 were disapproved:
- Antineoplastic drugs, skin and Ear Nose and Throat (ENT) drugs, circulatory system disease drugs, digestive system disease drugs, endocrine system disease drugs, anti-infective drugs and nervous system disease drugs accounted for 83% of the 1,310 approved chemical drug INDs. See figure 2 for more details.
Fig 2. Number of approved chemical drug IND in different indications
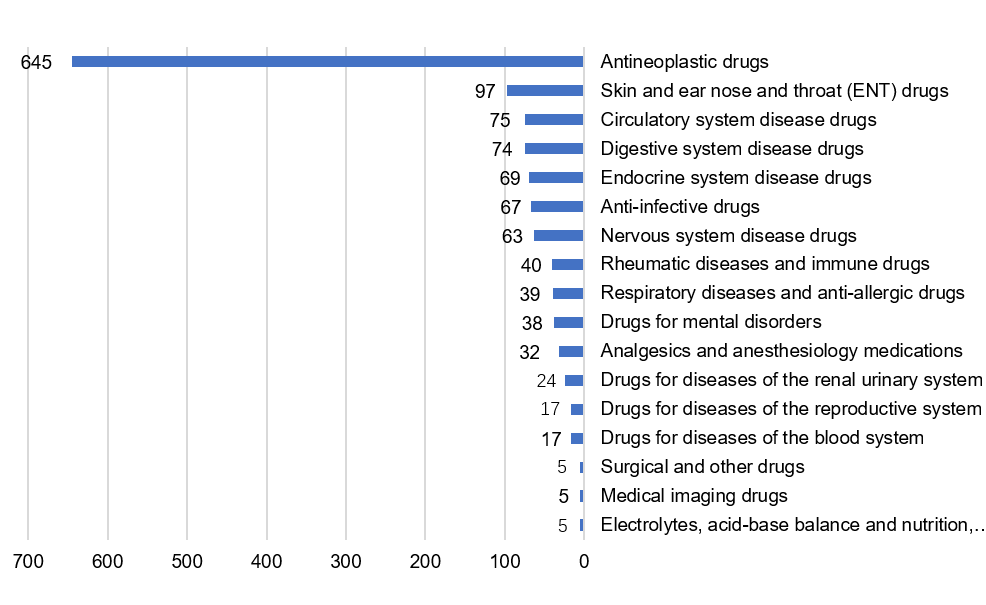
Among the 208 chemical drug NDAs, 160 were recommended for approval, and 8 were recommended for disapproval:
- Antitumor drugs, anti-infective drugs, nervous system disease drugs, circulatory system disease drugs, respiratory system disease drugs and antiallergic drugs, accounted for 73.75% of the 160 chemical drug NDA approvals. See Figure 30 of the report for more details.
Fig 3. Numbers of “recommended approval” chemical drug IND in different indications
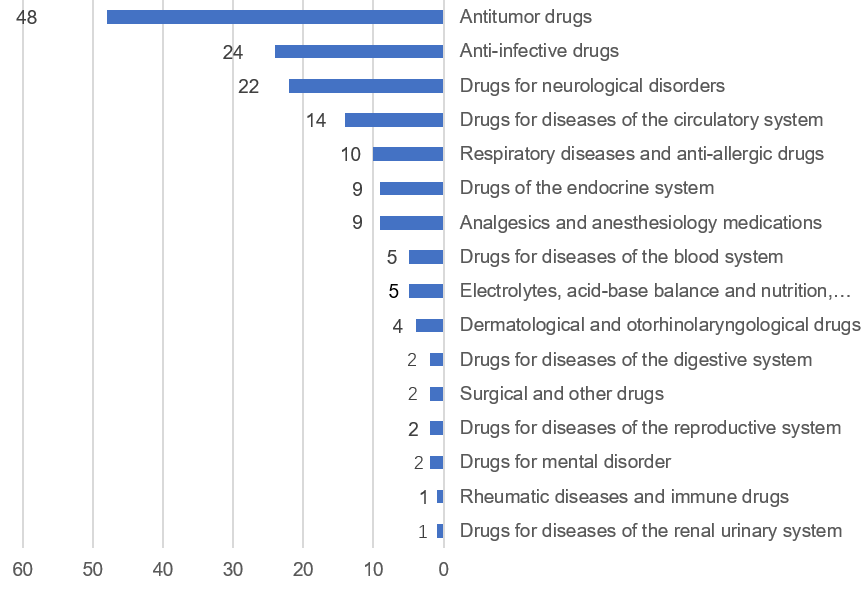
3. Biological product applications requiring technical review
3.1 Accepted biological product applications requiring technical review
In 2021, CDE accepted 1,999 biological product applications that needed technical review as shown in the table below:
| Biological product applications requiring technical review by category | Number of applications | |
|---|---|---|
| IND | Prophylactic biological products | 45 (including 26 INDs for 16 innovators) |
| Therapeutic biological products | 815 (including 617 INDs for 407 innovators) | |
| NDA | Prophylactic biological products | 13 (including 5 NDAs for 2 innovators) |
| Therapeutic biological products | 156 (including 18 NDAs for 14 innovators) | |
| In-vitro diagnostic (IVD) reagent | 9 | |
| Supplemental applications | 916 | |
| License renewal applications for overseas-manufactured biological products | 45 | |
3.2 Completed biological product registration applications requiring technical review
In 2021, the CDE has completed 1,920 biological product registration applications requiring technical review. In terms of registration application categories, there were:
Fig 4. Completed number of biological product registration applications in different categories in 2021
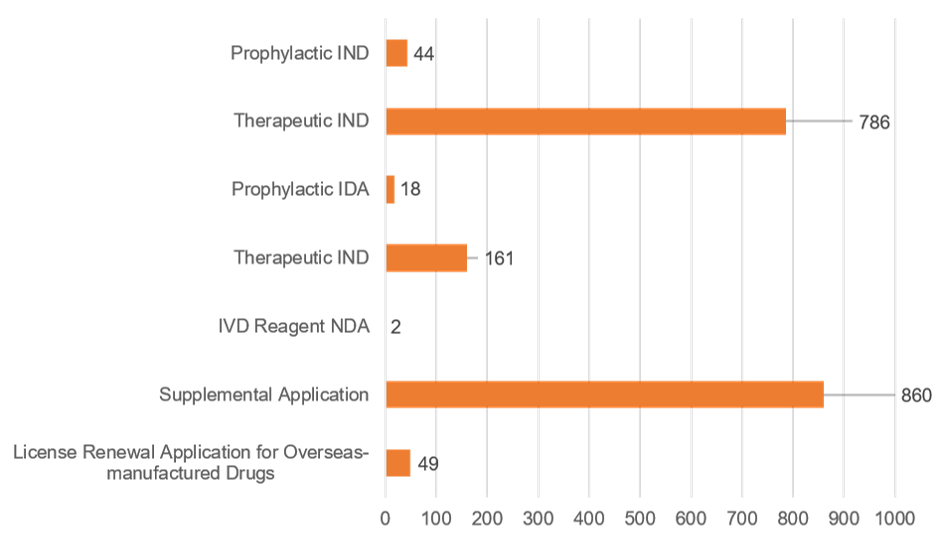
3.3 Approval/Recommended approval of biological product
As for biological products, the CDE approved 764 IND applications and recommended to approve 149 NDAs.
Biological product applications that needed technical review and were concluded in 2021 | ||||
|---|---|---|---|---|
| Application Type | Approval/Recommended Approval | Disapproval/Recommended Disapproval | Other | Total |
| Prophylactic IND | 44 | 0 | 0 | 44 |
| Therapeutic IND | 720 | 34 | 32 | 786 |
| Prophylactic IDA | 15 | 0 | 3 | 18 |
| Therapeutic IDA | 134 | 2 | 25 | 161 |
| IVD Reagent NDA | 0 | 2 | 0 | 2 |
| Supplemental Application | 787 | 6 | 67 | 860 |
| License Renewal Application for Overseas-manufactured Drugs | 48 | 0 | 1 | 49 |
| Total | 1,748 | 44 | 128 | 1,920 |
| * “Other” refers to the situations that review process were terminated because applicants did not pay the fees according to regulations, withdrew the application, etc. | ||||
4. Expedited approval
4.1 Breakthrough Therapy Designation (BTD)
In 2021, 53 applications for 41 products received BTD approval. The products’ indications were diseases caused by COVID-19 infections, non-small cell lung cancer, and ovarian cancer. Of the Recommended-to-be-approved NDAs five (5) were applications with BTD.
4.2 Conditional Approval (CA)
Among the 323 Recommended-to-be-approved NDAs, 60 NDAs for 38 products received marketing authorization through conditional approval.
4.3 Priority Review (PR)
In 2021, 115 applications for 69 products were admitted into priority review procedures. Among them, 41 applications were qualified for conditional approval; 34 applications were for paediatric drugs.
| Applications included in the priority review and approval process based on “Administration of Drugs Registration” | 2020 | 2021 | ||
|---|---|---|---|---|
| Registration application | Proportion | Registration application | Proportion | |
| Drugs urgently needed in clinical shortage; innovative drugs and improved new drugs for the prevention and treatment of major infectious diseases and rare diseases | 14 | 18.67% | 5 | 4.35% |
| Children’s drugs (new varieties, dosage forms and specifications) | 7 | 9.33% | 34 | 29.57% |
| Urgently needed vaccines and innovative vaccines for disease prevention and control | 4 | 5.33% | 3 | 2.61% |
| Drugs included in the breakthrough therapy program | – | – | 11 | 9.75% |
| Drugs met the requirement of approved with conditions | 27 | 36.00% | 41 | 36.65% |
| Others | 23 | 30.67% | 31 | 18.26% |
| Total | 75 | 100% | 115 | 100% |
4.4 Special approval (SA)
In 2021, 81 applications were granted special approval. They included:
- 5 NDAs (all with conditional approval), including 4 NDAs for inactivated COVID-19 vaccines (Vero cell) and 1 NDA for recombinant COVID-19 vaccine (adenovirus type 5 vector).
- 15 IND applications for therapeutic drugs against COVID-19 virus, including 4 for small molecular anti-viral drugs, 9 for neutralizing antibodies, and 2 for drug of other types.
- 5 NDAs for therapeutic drugs against COVID-19 virus. The drugs are Qingfei Paidu Granules, Huashi Baidu Granules, Xuanfei Baidu Granules, and Amubarvimab & Romlusevimab (BRII-196 & BRII-198) Combination Therapy.
More information
If you would like to know more about the drug registration process in China or our services for drug registration and China drug clinical trials please contact Cisema.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어