China medical device classification catalogue updates are crucial for manufacturers seeking market access and compliance. They can involve changes of risk category which impact new registrations as well as products already registered and available in China.
The National Medical Products Administration (NMPA) issued a notice on August 17, 2023, outlining adjustments to certain sections of the “Medical Device Classification Catalogue.” These changes encompass a range of devices, changing risk categories for certain products as well as introducing new products to the catalogue.
Key adjustments to the classification catalogue
The recent adjustments pertain to 58 categories of medical devices and encompass changes in product descriptions, intended uses, examples of product names, and classification management. More importantly, adjustments have been made to the risk management categories of the following medical device categories:
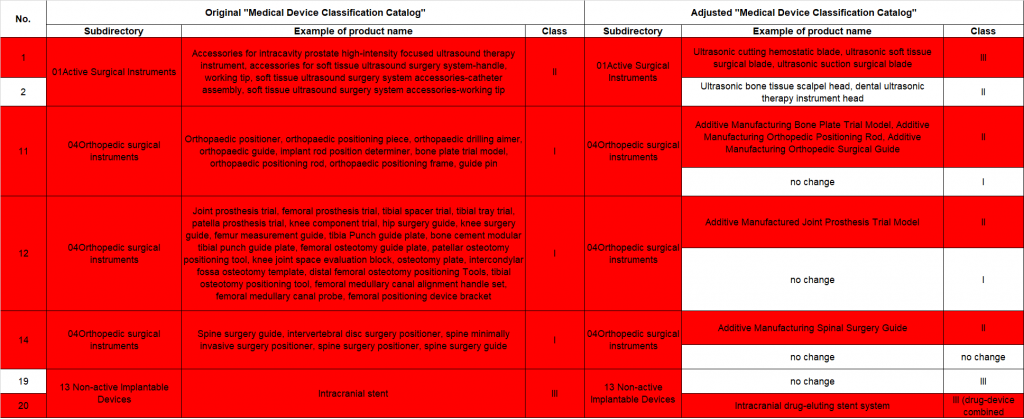
- Risk management category has increased (as highlighted in red in the following image) from Class I to Class II or from Class II to Class III, and should be paid special attention to. The current certificates of Class II are only valid until end of 2025, and should be apply for new risk classification as soon as possible.
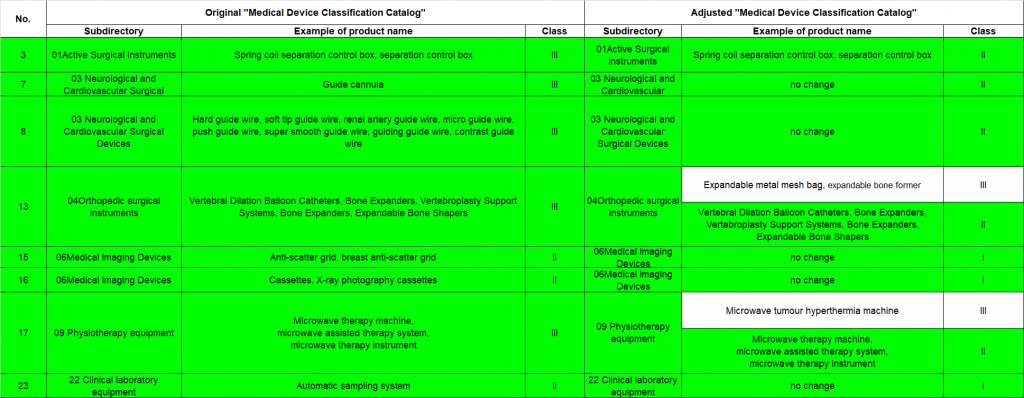
- Risk management category will be decreased (as highlighted in green in the following image) from Class III to Class II or from Class II to Class I where manufacturers will receive the certificate with updated reclassification upon renewal approval.
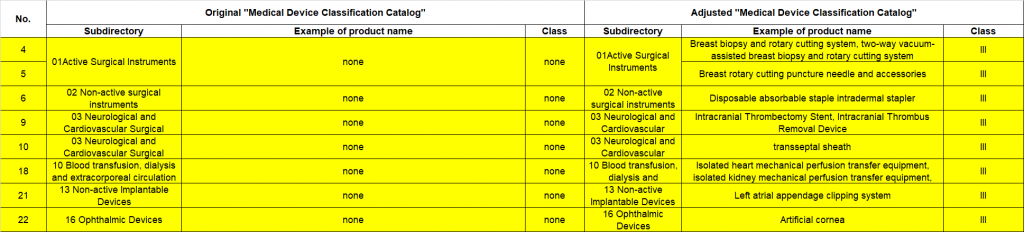
- Medical devices newly added to the classification catalogue (as highlighted in yellow in the following image).
Medical devices with new risk classification categories
The NMPA has laid out clear implementation requirements for the adjusted risk management categories:
- New applications for medical device registration will be accepted under the new categories from the date of the announcement
- Medical devices that were already in the process of registration under the previous categories will continue their evaluation and approval processes under those categories.
- For those who have obtained Class II medical device registration certificates, the existing product registrations will remain valid until December 31, 2025. The entities involved in these registrations are required to proactively initiate the process of converting their registration certificates in accordance with the relevant management category requirements. This conversion should be completed by December 31, 2025. For instance, already registered Class II medical devices that have had their risk category upgraded to Class III, the certificates are only valid until the end of 2025. Therefore manufacturers must apply for upgrades as soon as possible to avoid disruption in market access.
- For already registered medical devices that have their management category changed from Class III to Class II, the existing medical device registration certificate will remain valid within its validity period. If an extension is required, the registrant should apply to the respective MPA for registration renewal in accordance with the altered category. Upon approval, a medical device registration certificate will be issued with the adjusted product management category.
- For registered medical devices that have their management category changed from Class II to Class I, the medical device registration certificate will remain valid within its expiration period. Prior to the certificate’s expiration, registrants should apply for Class I filing with the respective Chinese regulatory authority.
Conclusion
The recent adjustments to the “Medical Device Classification Catalogue” by the NMPA signify a commitment to maintaining an up-to-date regulatory environment in the medical device industry. Manufacturers and regulatory professionals should be proactive in understanding these changes and adapting their strategies to align with the new classifications. By partnering with regulatory consulting experts, businesses can ensure a smooth transition and continued compliance within the ever-changing landscape of medical device regulations in China. Contact Cisema to understand the implications for your medical device registration and renewal.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어 



