China Medical Device Registrations 2023 Annual Report has been issued by the National Medical Products Administration (NMPA). Below we share some of the highlights.
Applications and approvals in 2023
In 2023, the NMPA received a total of 13,260 applications for initial registrations, registration renewals and changes in licensing items of Class III (Domestic and Overseas) and Class II (Overseas) medical devices. This represents a 25.4% increase when compared to 2022.
Of the 13,260 applications, the NMPA approved a total of 12,213 applications, an additional 278 products approved in comparison with the previous year.
Table 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs
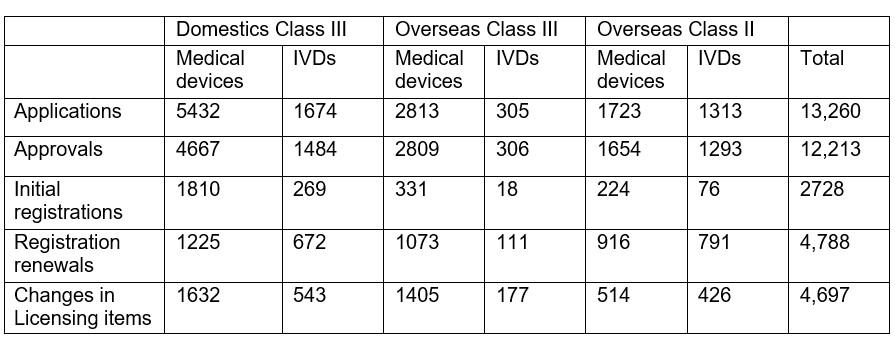
Chart 2. Percentage Distribution of Three Types of NMPA Approvals
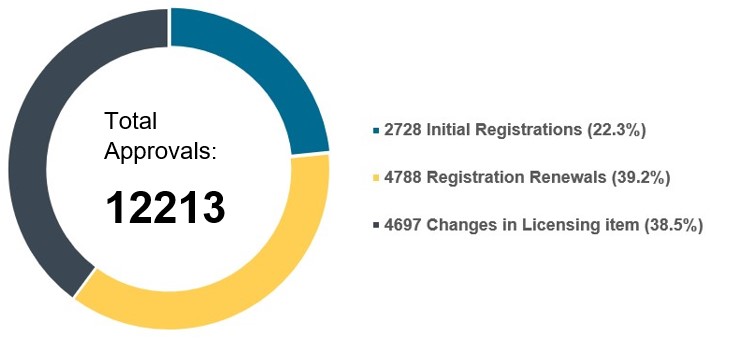
The split between product types remains the same as in 2022, with nearly 75% of all approvals for medical devices and the remaining 25% for IVDs.
Chart 3. Percentage Distribution of Approvals for Medical Devices and IVDs
Applications and approvals 2018-2023
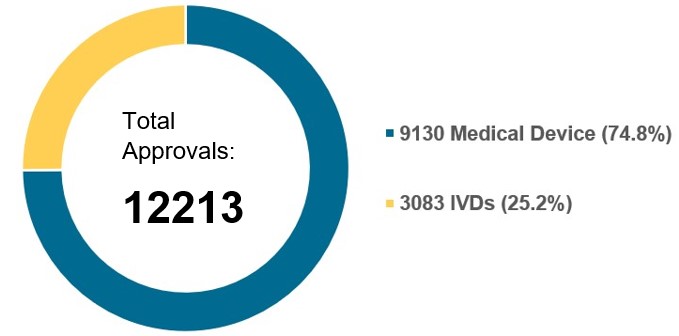
China’s NMPA accepted considerably more applications in 2023 following the slight slowdown in applications accepted in 2022. And China’s regulatory authority continues to approve more initial registrations, registration renewals and change applications year on year.
Chart 4. NMPA – No. of Registrations and Applications Approved from January 2018 to December 2023
Monthly breakdown of China medical device registrations during 2023
Chart 5. NMPA – No. of New Registrations Approved from January 2023 to December 2023
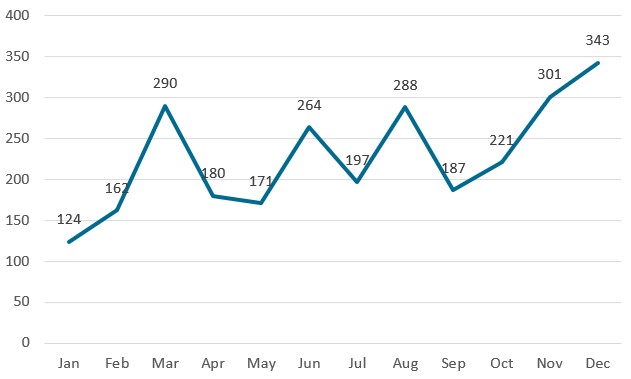
In 2023, the NMPA approved 2,728 new registrations in total.
Domestic Class I and Class II applications
Provincial Medical Product Administrations (MPAs) approved a total of 33,584 applications for China domestic Class II medical devices, an increase of 2.1% as compared to 2022.
Municipal MPAs handled a total of 25,681 filing applications for China domestic Class I medical devices, a decrease of 9.9% when compared to 2022.
Imported medical devices to China
Registered imported medical devices, except for in-vitro diagnostic reagents, cover products from 22 sub-categories in the Classification Catalogue of Medical Devices. The top five types of registered imported medical devices are:
- passive implantable devices
- stomatology devices
- ophthalmology devices
- medical imaging devices
- active surgical devices.
Compared to 2022, there have been significant changes, with ophthalmic instruments replacing infusion, nursing, and protective equipment. Active surgical instruments also replace neurological and cardiovascular surgical instruments.
The quantity of passive implantable devices increased considerably by 43.3% along with dental instruments increasing by 57.8%. However, the registration quantity of medical imaging devices decreased by 44.2% even though this category of products remains the fourth largest imported medical device by type.
China medical device imports by country
The United States of America, Germany, Japan, Korea and France continue to have the highest number of initial registrations for overseas medical devices as last year, totaling 77% of initial registrations for overseas medical devices.
List of Top 10 Countries Exporting Medical Devices to China
- USA
- Germany
- Japan
- South Korea
- France
- Italy
- Switzerland
- Ireland
- Sweden
- Israel
China Medical Device Fast-track Approvals
The NMPA received 466 applications for special review and approval of innovative medical devices in 2023. A total of 69 medical devices obtained special approval to market as Innovative Medical Devices in 2023.
From 2014 to 2023, the NMPA approved a total of 250 innovative medical devices manufactured by 167 domestic and 18 overseas enterprises, which means over a quarter were approved in 2023. This clearly demonstrates the increasing momentum for companies, both domestic and international, to go-to-market first in China thanks to the Innovative Medical Device pathway.
Further information
Read the NMPA’s Medical Device Annual Report for 2023.
If you think your medical device or IVD could benefit from the Innovative Medical Device pathway, please contact us.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어 



