China medical device standards 2023 annual report has been issued by the NMPA (National Medical Products Administration). It provides a comprehensive overview of the standards that were developed during 2023.
Total medical device standards in effect
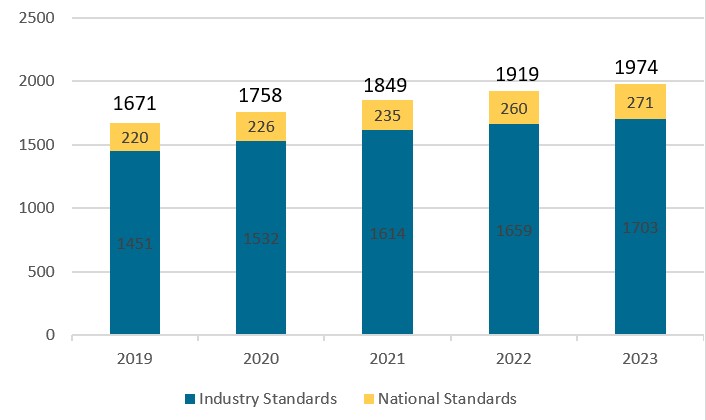
A total of 1,974 standards were in effect by the end of 2023 for medical devices, covering 275 compulsory standards and nearly 1700 recommended ones. Please refer to Table 1 below.
National standards are represented by the letters GB whereas medical device industry-specific standards are represented by YY. Standards can also be designated as compulsory or recommended, the latter is represented by “/T” following the initial letters.
Table 1. Medical device standards in effect at the end of 2023
| Compulsory | Recommended (/T) | Guidance technical documents | Total | |
| National Standards (GB) | 95 | 171 (GB/T) | 5 | 271 |
| Industry Standards (YY) | 180 | 1523 (YY/T) | / | 1703 |
| Total | 275 | 1694 | 5 | 1974 |
In comparison to previous years, the Year-on-Year (YoY) growth in the total number of medical device standards is slowing down to around 3% per annum from 5% back in 2019-2020. Please refer to Chart 2 below. This reveals continued progress and initiatives by the NMPA to enhance the quality and effectiveness of regulations governing the medical device industry.
Chart 2. Medical device standards in effect from 2019-2023
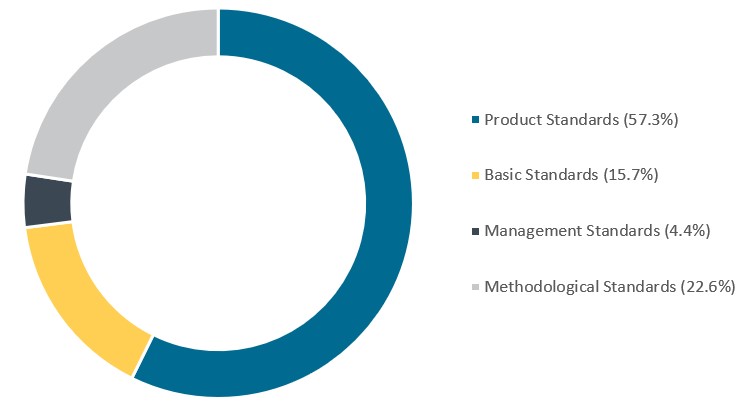
The vast majority of standards that are in place are product-specific standards at 57%, with management standards representing the least at 4%.
Chart 3. 2023 medical device standards types distribution
New standards introduced or modified in 2023
In terms of standard approval and publication, both national and industry standards saw significant advancements. The NMPA approved and released 28 national standards and 131 industry standards, with a notable focus on recommended standards. This emphasis underscores the importance of flexibility and industry collaboration in addressing the dynamic landscape of medical device technology and practices.
International standards in China
International standards are closely tracked and researched by the NMPA, and those that are suitable for transformation into China are typically transformed within two (2) years after their release. In 2023, a total of 86 international standards for medical devices were approved and issued for transformation into national standards or industry standards in China, with a consistency rate of over 90%.
Why is it important to keep abreast of standards updates?
Compliance with and regulatory control of both GB and YY standards is governed by the “Regulations for the Supervision and Administration of Medical Devices”. The various standards have guiding significance for writing the performance indicators of the Product Technical Requirements (PTR) which is an integral part of medical device registrations in China. A declared product must meet mandatory standards, whereas the recommended standards are product-specific.
If standards are updated or new standards are introduced then a declared product must prove that it meets these standards. Normally, a lead time to adapt to the new standard will be announced by the NMPA for medical devices that are already registered and on the market in China. During this lead time, it is important to perform the “type-testing” again if required by the standard update/introduction.
It is important to pay special attention to Chinese mandatory standards and their updates. Since October 1, 2021, it is no longer permitted to submit a product change and certificate renewal at the same time. If a product change requires type testing (e.g. due to standards updates or new standards being introduced), then a product change application should be submitted separately before the renewal registration application is made. Otherwise, the certificate will not be renewed as scheduled, which may lead to the severe consequence that your products can’t be imported or those already in China may no longer be sold.
Innovative products with no corresponding standards
As China encourages the registration of innovative medical devices through its Innovative Medical Device Pathway, such products may not have currently applicable national or industry standards. In such instances, production, safety or quality standards referencing existing ISO standards or other official standards may be conducive to registration.
Further information
Click here to read the full official announcement about on the China Medical Device Standards 2023 Annual Report.
To check if your device is affected by standards update, please contact Cisema.
Request our whitepaper on NMPA Approvals of Medical Devices for a comprehensive overview on filing, registering and maintaining compliance in China.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어