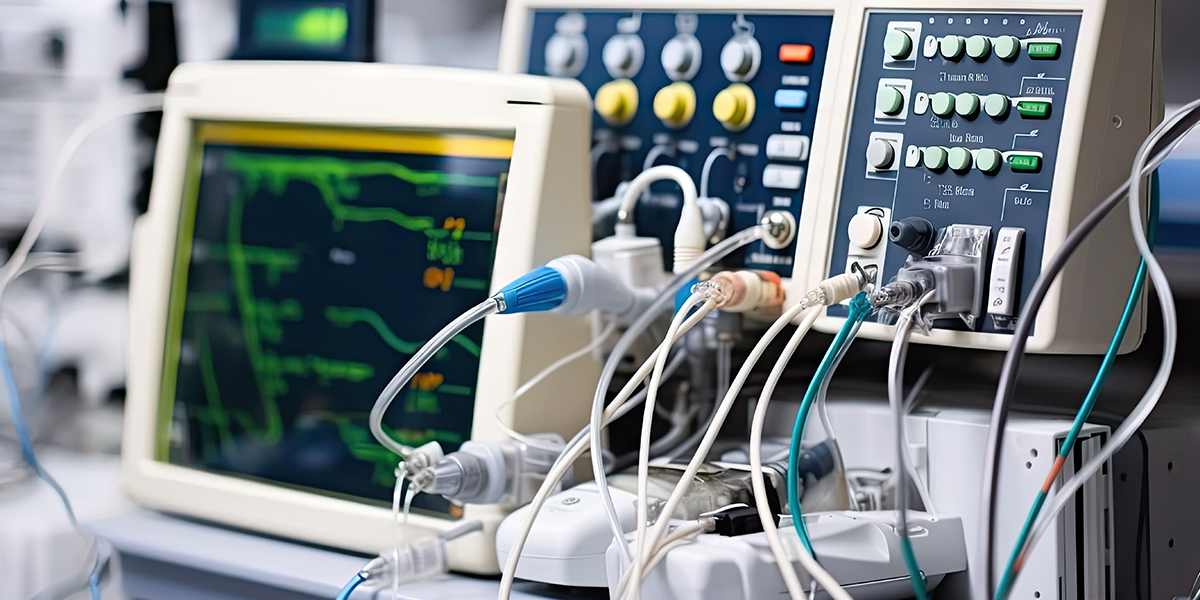
China Medical Device Standards Catalogue 2024 has been released by the National Medical Products Administration (NMPA), announcing a total of 1974 medical device standards.
The Standards Catalogue is divided into two main parts, the first of which covers quality management of medical devices, Unique Identification of Medical Devices (UDI) and medical device packaging.
The second part covers different categories such as Biological Evaluation, Electrical Devices, Disinfection and Sterilisation, Surgical Devices, Surgical Implantable Devices etc.
The Catalogue lists the standard serial number, standard number, standard name, date of publication, date of implementation, substitution relationship (applicable to standards that have been published but not yet implemented), the unit of responsibility, and the scope of application. In this article, we list the standards that will come into effect in 2024.
| Serial number | Standard number | Standard name | Release date | Date of implementation | Alternate relationships (applicable to standards that have been published but not yet implemented) |
| 727 | YY 1045-2021 | Dentistry Handpieces and Motors | 2021-09-06 | 2024-05-01 | YY 1045.1-2009、YY 1045.2-2010、YY 0836-2011、YY 0837-2011 |
| 1228 | YY 9706.247-2021 | Medical Electrical Equipment Part 2-47: Specific requirements for basic safety and essential performance of Holter systems | 2021-09-06 | 2024-05-01 | Instead of YY 0885-2013 |
| 1247 | YY 9706.234-2021 | Medical Electrical Equipment Part 2-34: Specific Requirements for Basic Safety and Basic Performance of Invasive Blood Pressure Monitoring Devices | 2021-09-06 | 2024-05-01 | Instead of YY 0783-2010 |
| 1272 | YY 9706.221-2021 | Medical Electrical Equipment Part 2-21: Basic Safety and Basic Performance Specific Requirements for Infant Radiant Warmers | 2021-09-06 | 2024-05-01 | Instead of YY 0455-2011 |
| 1276 | YY 9706.252-2021 | Medical Electrical Equipment Part 2-52: Specific requirements for basic safety and essential performance of medical beds | 2021-09-06 | 2024-05-01 | YY 0571-2013 |
| 1279 | GB 9706.204-2022 | Medical Electrical Equipment Part 2-4: Specific requirements for basic safety and essential performance of cardiac defibrillators | 2022-07-13 | 2024-08-01 | GB 9706.8-2009 |
| 1417 | YY 9706.270-2021 | Medical Electrical Equipment Part 2-70: Specific Requirements for Basic Safety and Basic Performance of Sleep Apnea Treatment Devices | 2021-09-06 | 2024-05-01 | YY 0671.1-2009 |
| 1418 | YY 9706.272-2021 | Medical Electrical Equipment Part 2-72: Specific requirements for basic safety and essential performance of home ventilators used by patients dependent on ventilators | 2021-09-06 | 2024-05-01 | YY 0600.2-2007 |
| 1553 | GB 9706.222-2022 | Medical Electrical Equipment Part 2-22: Specific requirements for basic safety and essential performance of laser devices for surgical, orthopedic, therapeutic, and diagnostic use | 2022-03-15 | 2024-05-01 | GB 9706.20-2000 |
Further information
Read the original NMPA announcement on China medical device standards catalogue 2024 update.
Read our recently published articles on China medical device standards updates:
- New medical device industry standards approved in June 2023 by the NMPA
- China medical device industry standards March 2023 update
- China GB9706 Medical Electrical Equipment Standards Expert Consultation Mechanism is launched
Discover our services for medical device registration, renewals and NMPA Legal Agent.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어