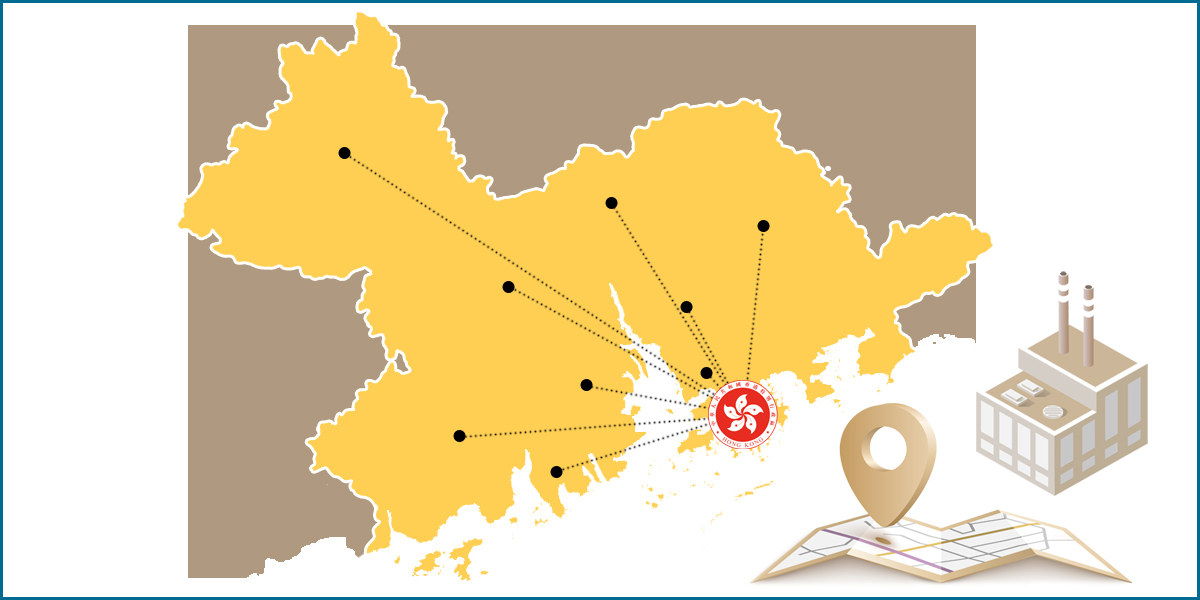
Contract manufacturing of Hong Kong medical device and drug in GBA (Greater Bay Area) is now supported by the China NMPA (National Medical Products Administration) following its implementation plan announced on June 29, 2022. The implementation plan supports Hong Kong and Macao medical device and drug market authorization holders (MAHs) to entrust cross-border production in the 9 cities of the Greater Bay Area, which includes Guangzhou, Shenzhen, Zhuhai, Foshan, Huizhou, Dongguan, Zhongshan, Jiangmen and Zhaoqing.
Highlights
Scope of Application
The plan is applicable to proprietary Chinese medicines, chemical drugs and medical devices that fulfill the following conditions:
| Scope | |
|---|---|
| Drug | Medical Device |
| |
| |
|
|
|
|
Steps to contract manufacturing of Hong Kong medical device and drug in GBA
| Application Steps | ||
|---|---|---|
| # | Drug | Medical Device |
1 |
| |
2 |
| |
3 |
|
|
4 |
|
|
Responsibilities of MAH, domestic agent and contract manufacturers for drugs
- The Hong Kong / Macao MAH shall ensure the quality management of the entire product life cycle is well established, and the obligations for drug traceability, pharmacovigilance, annual reports, etc. are performed.
- The Hong Kong / Macao MAH shall supervise the contract manufacturers to ensure the obligations stipulated in the quality agreement and the entrustment agreement are being fulfilled.
- The contract manufacturer shall ensure the production process, quality standards and GMP is in strict accordance with the registration approval requirements, and must keep all documents and records.
- The domestic agent shall perform the obligations of the MAH and bear joint liability with the MAH
Responsibilities of MAH, domestic agent and contract manufacturers for medical devices
- The Hong Kong / Macao MAH shall ensure the quality management of the entire product life cycle is well established, and the obligations for adverse event monitoring and product recall, etc. are performed.
- The Hong Kong / Macao MAH shall supervise the contract manufacturers to ensure the obligations stipulated in the quality agreement and the entrustment agreement are being fulfilled.
- The domestic agents shall bear joint liability whilst assisting Hong Kong / Macao MAH in fulfilling their registrant obligations.
- If the production and business operation in the 9 GBA cities violates the Supervision and Administration of Medical Devices or other related regulations in China, the Hong Kong / Macao MAH shall bear the main responsibility whilst domestic agents bear joint liability and contract manufacturers bear the corresponding legal responsibility.
Further information
If you would like to learn more information about the GBA pathway for medical device and pharmaceutical products, please contact Cisema.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어