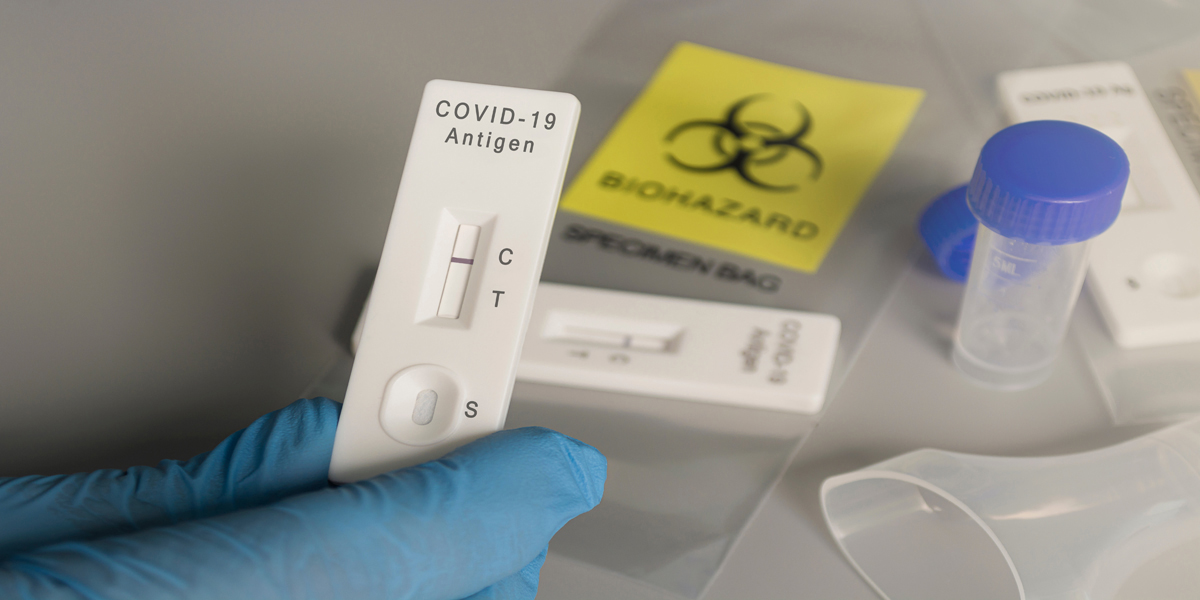
Coronavirus product manual changes have been introduced by the National Medical Products Administration (NMPA) in China.
The NMPA has recently issued Notice No. 349 of 2023, which outlines important modifications to product manuals for Coronavirus nucleic acids, antigens, and antibodies detection reagents.
Revised Guidelines and Streamlined Content:
The NMPA has revised and issued three coronavirus diagnostic product guidelines, specifically focusing on the registration and review of Nucleic Acid Detection Reagents for Novel Coronaviruses (2019-nCoV). The key areas of content that have been streamlined include the instructions, specifications, and user manuals for these detection reagents. The modifications encompass elements such as intended use, sample requirements, inspection methods, interpretation of test results, and limitations of the inspection method.
Modifications without Change of Registration or Filing:
It is important to note that if the product itself remains unchanged, along with its performance, registrants are required to modify the product specification in accordance with the content specified in the Annex of Notice No. 349 of 2023. This modification can be carried out without applying for a change of registration or filing. However, during the renewal of registration, the revised product manual should be provided to the regulatory authorities.
Guidelines Compliance:
The NMPA’s revisions align with the New Coronavirus Infection Diagnosis and Treatment Programme issued by the National Health and Health Commission. These revisions have adjusted the expressions of intended use and modified the relevant contents of the instruction manuals for new coronavirus nucleic acid, antigen, and antibody detection reagents. Therefore, it is essential for regulatory affairs managers to review and align their product manuals with the updated guidelines.
Handling Other Changes:
In cases where other changes occur to the product, regulatory affairs managers must adhere to the relevant requirements outlined in the Administrative Measures for the Registration and Filing of In Vitro Diagnostic Reagents. Any alterations beyond the scope of the revised guidelines may necessitate additional procedures and documentation.
Further Information:
Read the original article on Coronavirus product manual changes required for China.
If you are intending to register IVDs in China including for Coronavirus detection or see if these Coronavirus product manual changes apply to your product and obtain advice on how to implement them, please contact Cisema for a quote.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어