From January 1, 2023, onwards, the safety information of all ingredients will be required for filing and registering cosmetics in China. Based on the Regulations on the Management of Cosmetics Registration and Filing Documents, the ingredient submission code will be a replacement of the ingredient safety information and a new trend requirement under the CSAR in China. The material supplier company could choose to register an ingredient submission code to replace numerous ingredient safety documents.
| Ingredient Submission Code | |
| Scope | For all cosmetic raw material in IECIC |
| Applicant | Cosmetic raw material manufacturers |
| Purpose | Receive the ingredient submission code and provide it to cosmetics companies |
Highlights
With the introduction of the CSAR in January 2021, the raw material suppliers are affected by the transitional implementation of new requirements between May 2021 and May 2023.
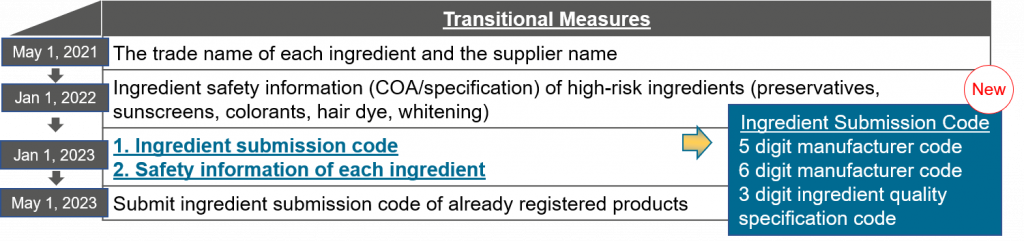
After the raw material safety information from the supplier is submitted in Chinese through the online platform designated by the NMPA (National Medical Products Administration), the ingredient submission code will be generated. The submission of ingredient submission code will be mandatory from 2023, however, the online platform is yet to be announced for launch. Read our blog post on the CSAR related action timeline for further information.
By Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어