On March 5, 2021, the NMPA (National Medical Products Administration) announced the following measures for cosmetic registration (of special cosmetics) and filing (of general cosmetics) information management to support the implementation of the CSAR (Cosmetic Supervision and Administration Regulation):
1. New cosmetics registration and filing online platform
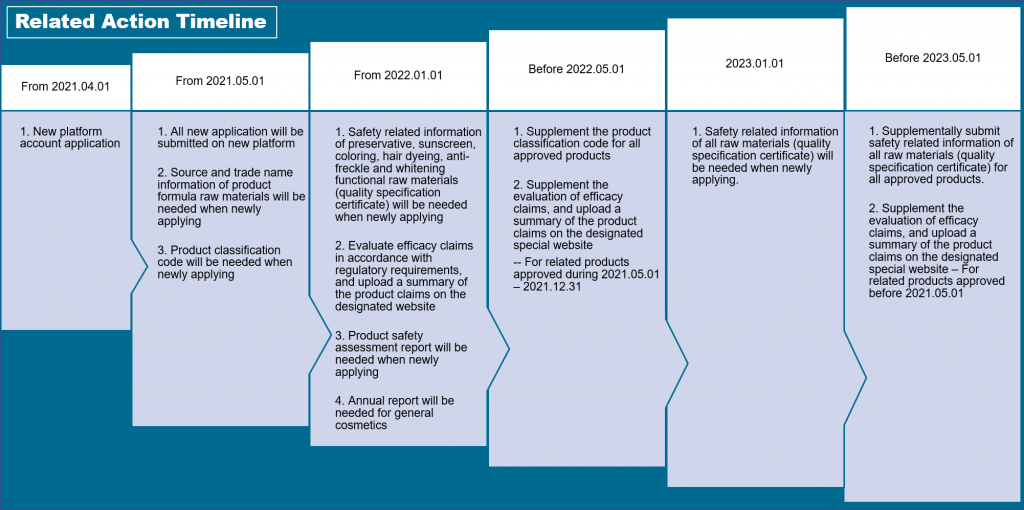
From April 1, 2021, onwards, the online platform for cosmetics registration and filing (https://zwfw.nmpa.gov.cn) is available for domestic applicants or DRAs (Domestic Responsible Agents) of overseas applicants to open an account.
From May 1, 2021, onwards, the online platform will allow the submission of cosmetics registration and filing materials.
In addition, the older version of the online platform will only be handling applications submitted before May 1, 2021. Any new applications will not be accepted after May 1, 2021.
2. Products already registered or filed in the older version of the online platform
For cosmetic products that obtained the registration or filing approval through the older version of the online platform, the applicants are responsible to submit the following information and materials to the new online platform by May 1, 2022:
- Applicable product technical standards covering national standards, industry standards, etc.
- Sample of the product label
- Product formula for domestic general cosmetics
- Picture of the product packaging
3. Submission of information on the safety of cosmetic raw materials
From May 1, 2021, onwards, the source and the name of all raw materials (used in the product formula) should be submitted as part of the registration or filing of cosmetics. If the product contains raw materials that are regulated under the technical specifications for cosmetic safety, the proof of meeting quality standards and the product safety information should be submitted as well.
From January 1, 2022, onwards, the product safety information of raw materials with the functions for antiseptic, sun protection, hair coloring, freckle removal, and whitening should be submitted for the registration or the filing of cosmetics.
The product safety information of all raw materials used in the cosmetic products should be submitted for any registration or filing applications after January 1, 2023. For cosmetics that have been registered or filed before January 1, 2023, the product safety information of all raw materials shall be provided by May 1, 2023.
The new concept of raw material submission codes is also being introduced. This digital code is composed of a five-digit manufacturer code, a six-digit raw material code, and a three-digit raw material quality specification code. It is automatically generated when the cosmetic raw material manufacturer submits the ingredient safety information through the online platform designated by NMPA. The manufacturer would then provide these raw material submission codes to the cosmetic applicants, who can then fill in these codes to associate the raw material safety information without the need to upload related documents by themselves.
4. Test report on the cosmetic efficacy claims of anti-freckle, whitening and anti-hair loss
The human efficacy test report should be submitted for any registration applications after January 1, 2022.
For any registrations of anti-freckle, whitening and anti-hair loss cosmetics applied and approved before May 1, 2021, the registrant should submit the human efficacy test report before May 1, 2023.
For any registrations submitted and approved between May 1, 2021, and December 31, 2021, the registrant should submit the human efficacy test report before May 1, 2022.
5. Annual report on general cosmetics
From January 1, 2022, onwards, all general cosmetics that have been filed for a year should submit the annual report to the new online platform between January 1 and March 31 every year.
By Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어