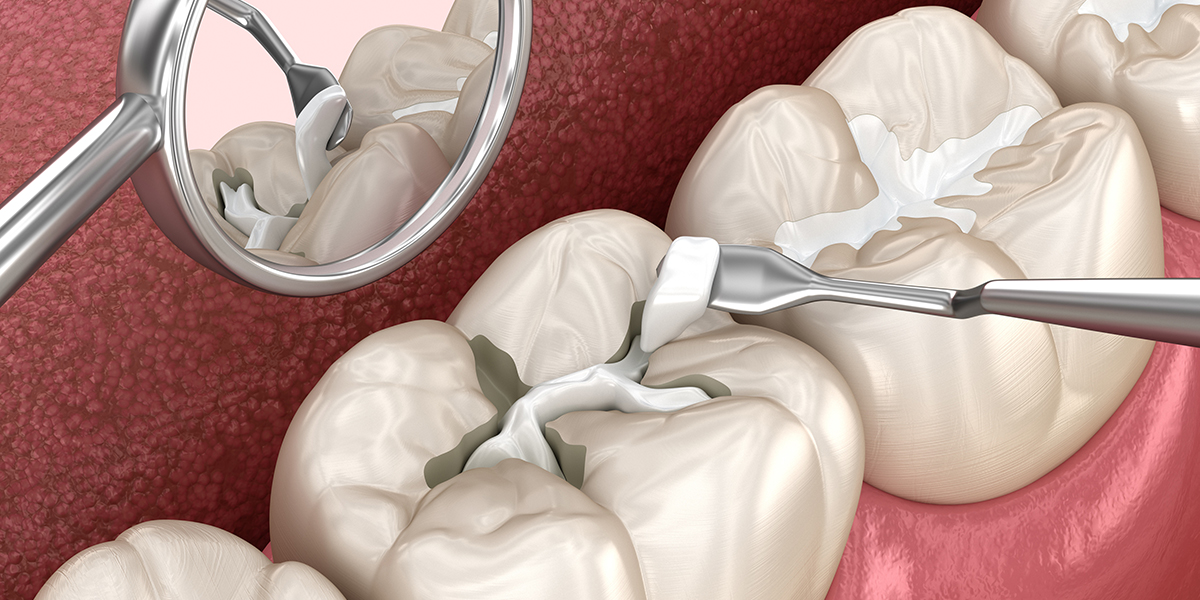
Dental adhesive products registration review guidelines for China came into effect on March 7, 2023.
These new guidelines issued by China’s Center for Medical Device Evaluation (CMDE) apply to dental adhesive products for dental filling and restoration, as well as restoration and bonding. However, it’s important to note that products with special designs and innovations are not covered under this guideline.
In terms of registration review points, there are several key regulatory information requirements to keep in mind. First, the naming of products must adhere to the Medical Device Common Name Naming Rules, General Name Naming Guidelines for Dental Instruments, and other relevant regulatory requirements. The product name should use the Medical Device Classification Catalog or national standards, industry standards for the common name, or the product’s clinical intended use, curing mode, and applicable part as the basis for naming.
Additionally, according to the “Medical Device Classification Catalogue,” the classification code for dental adhesive products is 17-05-02, and the risk management category is class III.
When it comes to the division of product registration units, the guidelines recommend using technical principles, structural composition, performance indicators, and scope of application as the basis for division. Any division of product registration units must comply with the requirements of the “Guiding Principles for the Division of Medical Device Registration Units”.
It’s worth noting that different products may have different registration units based on their main chemical composition, bonding resin matrix and filler components, or curing principle. For example, light-curing adhesives, chemical curing adhesives, and dual-curing resin adhesives may need to be divided into different registration units. On the other hand, bonding agents that must be used jointly to play a bonding role can be used as the same registration unit.
Overall, following these new dental adhesive products registration review guidelines is essential to ensure the smooth registration and renewal of dental adhesive products. If you have such products, it’s recommended that you prepare your registration documents in accordance with the requirements of the review guidelines. Doing so will make the registration process smoother and help you avoid potential delays or issues.
Further information
Read the CMDE full registration review guidelines on dental adhesive products.
Are you looking to expand your medical device business to China? Don’t let the complex registration process hold you back! Discover our expert medical device registration services for China today.
Our team of experienced professionals understands the unique requirements and regulations for medical device registration in China, and we can guide you through the process from start to finish. With our comprehensive services, you can ensure your medical devices meet all necessary standards and are ready for market in China.
Don’t wait any longer to tap into the vast potential of the Chinese medical device market. Contact us today to learn more about our registration services and take the first step towards success in China!

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어