GB9706 deadlines have been updated by the NMPA. GB9706.1 is the Chinese standard applicable to medical electrical equipment in China. The letters GB signify that it is a national standard. It largely mirrors the IEC 60601-1 standard. However, the effective version in China has often lagged behind IEC versions, leading to some testing and compliance difficulties for international companies in the China market.
Most recently, the update to the 2020 version, which aligns with the 2012 IEC standard, has led to much industry consternation because it necessitates product re-testing in China to prove compliance with the newly updated standard (GN9706.1-2020). Industry has been slow to react because of the huge number of devices affected. This article discusses the latest status of the GB update and suggests actions to ensure continuing compliance in China.
However, there are some product-specific sub-standards for GB9706.1-2020, such as:
- YY9706.269-2021
- YY9706.221-2021
- GB9706.218-2021
- GN9706.202-2021
which have their own specific implementation dates. For the 9706 series of related standards, there is a transition period of 2 and 3 years respectively, to make the corresponding changes for Class I, II and III medical device standards with implementation dates before December 31, 2025. For the 9706 series of standards whose implementation date is after December 31, 2025, it should complete the corresponding change registration/ filing before the implementation date.
Timeline of implementation
The transition to the new standard follows a structured timeline set out here:
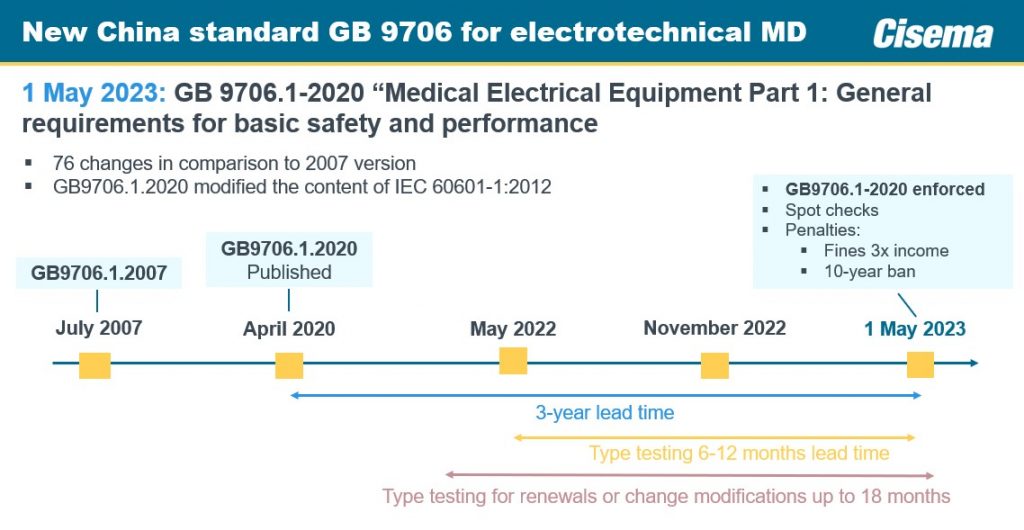
- July 2007: GB9706.1-2007 established the baseline standard
- April 2020: GB9706.1-2020 was published, providing a three-year lead time for implementation
- March 16, 2023:The NMPA granted extensions for products to implement the new standards:
- 3 years extension for change of registration of Class II and Class III medical devices
- 2 years extension for change of filing of Class I medical devices
- May 1, 2023: Enforcement of GB9706.1-2020 begins with spot checks.
Penalties for non-compliance may include fines of up to three times the income and bans for ten years.
Ensuring compliance: Change approval process
For standards with implementation dates before December 31, 2025, product registrations and filings should be handled as follows:
- For Class II and Class III medical electrical equipment applying for registration for the first time, manufacturers must submit NMPA inspection reports compliant with the new standards. Previously submitted applications, accepted before, can undergo NMPA inspection, review, and approval based on the original standards.
- For Class II and Class III medical electrical equipment that is already registered, manufacturers should promptly apply for registration changes, submit NMPA inspection reports compliant with the new standards, and complete product registration changes according to the new standards within three years from the implementation date specified in the first provision of the relevant standards.
- For Class I medical electrical equipment filing for the first time, manufacturers should submit NMPA inspection reports compliant with the new standards during product filing.
- For Class I medical electrical equipment that is already filed, the deadline for filing changes is April 30, 2025. During filing changes, manufacturers must submit inspection reports compliant with the new standards.
For specialized standards with implementation dates after December 31, 2025, registered or filed medical electrical equipment must complete product registration changes or filing changes before the implementation date specified in the first provision of the relevant standards.
Non-compliance with GB9706.1-2020
Failure to comply with the updated GB9706.1-2020 and corresponding specialized standards poses significant risks, especially given the Chinese government’s increasingly stringent enforcement through post-market surveillance, random sampling tests, and addressing adverse events related to device use.
How can Cisema help?
Cisema offers tailored solutions, including type test reports, gap analysis, document review, evaluation of standards, and consultation. With our support, manufacturers can obtain type test reports aligned with IEC standards and positive GAP analyses comparing IEC standards to those in China.
Further information
For further detailed information, please refer to the NMPA announcements:
- https://www.nmpa.gov.cn/ylqx/ylqxggtg/20230314143850152.html
- https://www.nmpa.gov.cn/xxgk/zhcjd/zhcjdylqx/20230315155652197.html
- https://www.nmpa.gov.cn/xxgk/zhcjd/zhcjdylqx/20230407175156186.html
Compliance with evolving regulatory standards is vital for medical device businesses. With our expertise, navigating the transition to GB9706.1-2020 becomes smoother, ensuring your products meet top safety and performance standards. Contact us to learn more about how we can support your compliance efforts.
GET IN TOUCH

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어