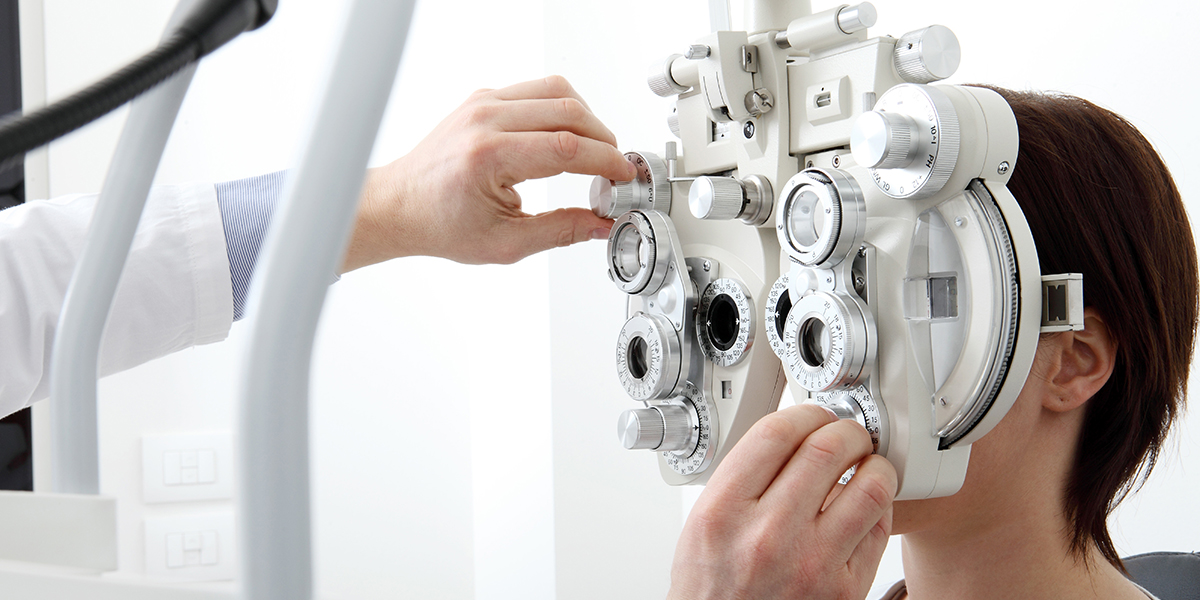
China registration of ophthalmic optical measurement devices shall refer to the respective technical review guidelines issued (No.13 of 2023) by the Centre for Medical Device Evaluation (CMDE) under the National Medical Products Administration (NMPA) on April 28, 2023.
When it comes to registering ophthalmic optical measuring equipment, it has many challenges for manufacturers to register. These guidelines aim to assist applicants in preparing and submitting registration documents for technical evaluation of ophthalmic optical measurement devices. They also serve as a reference for regulatory authorities in reviewing the submitted materials.
Scope of Application
These guidelines specifically apply to diagnostic/measurement devices that utilize optical imaging/measurement principles to obtain ophthalmic biological parameters. These devices fall under the category of Class II medical devices as per the 2017 Medical Device Classification Catalogue. and encompass products with classification codes 16-04-13, 16-04-14, and 16-04-15. Other medical devices that utilize optical principles to obtain anterior segment biological parameters can also refer to these guidelines for applicable sections. It is important for applicants to review the guidelines and determine their applicability based on the specific characteristics of their products.
Key Points
The guidelines encompass various essential aspects that need to be addressed during the registration review process. Here are the key points covered:
- Regulatory Information:
- Applicants need to provide accurate and detailed information regarding the product name and division of registration units. The latter should be based on the different working principles and imaging/measuring principles employed by the equipment.
- Summary:
- A comprehensive overview of the product structure, including its main components (host, display unit, operating unit, lighting unit, chinrest, forehead rest) and functions, should be provided.
- Additionally, the working principle of the equipment and its differences from similar products in the market need to be explained.
- The intended scope of application and the specific environment for its use must be clearly stated.
- Product Risk Management Data:
- Applicants should identify and evaluate potential risks associated with their measuring equipment. This includes energy hazards, biological hazards, environmental hazards, hazards related to use, software hazards, and more. A risk analysis management report must be prepared to demonstrate how risks are controlled and reduced.
- Basic Principles of Safety and Performance:
- The guidelines outline the basic principles of safety and performance that must be followed. Applicants need to refer to the Technical Guidelines for Compliance with the Basic Principles of Safety and Performance of Medical Devices and provide detailed information on how their product complies with each requirement.
- Product Technical Requirements and Inspection Report:
- Detailed product technical requirements, including performance parameters and specifications, should be prepared in accordance with the guidelines. An inspection report highlighting electrical safety, electromagnetic compatibility, laser light source requirements (if applicable), and more should be submitted.
- Research Data:
- Various research data needs to be provided to support the product’s performance claims. This includes product performance research, software and network security research, study on biological characteristics, cleaning and sterilization research, stability study, optical radiation safety research data, and other relevant information.
- Clinical Evaluation Requirements:
- The guidelines specify that ophthalmic optical measurement devices, except for corneal pachymeters (16-04-13), do not fall under the category of devices exempt from clinical evaluation. Therefore, applicants must conduct clinical evaluations based on the structure, performance parameters, and intended use of the products. The relevant requirements from the “Quality Management Specification for Clinical Trials of Medical Devices” and the “Technical Guidelines for Clinical Evaluation of Medical Devices” should be considered.
- Sample of Instructions and Labels:
- Product instructions and labels must comply with relevant regulations and guidelines. The guidelines provide detailed instructions on what information should be included on the labels and in the instructions, covering aspects such as warnings, maintenance instructions, and software-related information.
- Comparative Evaluation for Devices with Additional Functions:
- For devices that not only obtain ophthalmic biological parameters but also have multiple functions and qualify for exemption from clinical evaluation, a comparative evaluation approach can be adopted. For example, if a device can measure corneal topography and obtain parameters such as corneal curvature and refractive power through non-contact methods, it can be compared with the corneal topography product listed in the exemption catalog (16-04-12) to demonstrate equivalence. Similarly, devices with functions like diagnosing dry eye using tear film interferometry or capturing ocular images can be compared with products such as dry eye detectors (16-04-19) or ophthalmic cameras (16-04-05) to establish equivalence.
- Choosing the Appropriate Clinical Evaluation Pathway:
- The guidelines also recommend that applicants consider the risks and benefits of ophthalmic optical measurement devices and select an appropriate clinical evaluation pathway to demonstrate their safety and effectiveness in clinical applications. This approach ensures that the evaluation process aligns with the device’s intended use and provides comprehensive evidence to support regulatory compliance.
The guidelines serve as a valuable reference for regulatory consultants, technical review departments, and registration applicants involved in the registration of ophthalmic optical measuring equipment in China. It is crucial to follow these guidelines, along with applicable regulations, to ensure a successful registration process. As regulations and standards continue to evolve, it is essential to stay updated and make necessary adjustments to comply with future requirements.
Further information
Read the original CMDE guidelines for registering ophthalmic optical measuring equipment in China.
Discover our services for medical device registration, renewals and NMPA Legal Agent.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어