Medical device classification catalogue, the extensive list specifying risk classes for various product categories in China, has been updated twice in March 2022.
On March 24, 2022, the National Medical Products Administration (NMPA) announced (No.25-2022) the changes for 10 medical device product categories. On March 30, 2022, the NMPA announced (No.30-2022) the changes for 27 medical device product categories.
Medical Device Classification Catalogue Changes – Highlights
The key changes to China’s medical device classification catalogue have been summarized by our regulatory experts as follows.
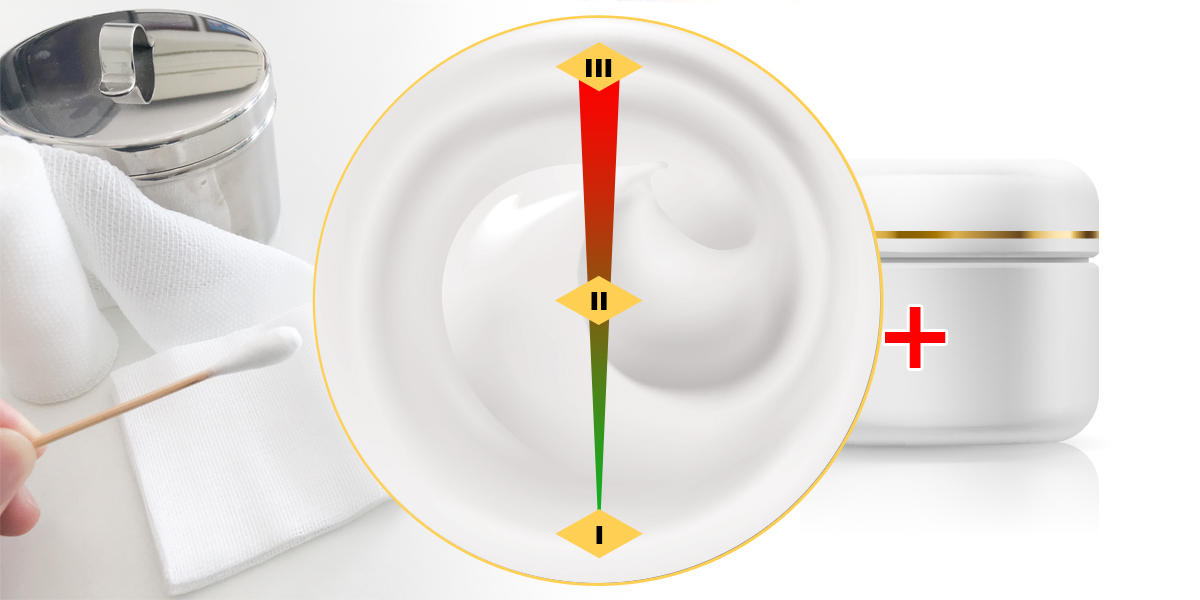
- Non-sterile types of liquid and paste dressings will be upclassified as class II medical devices instead of class I.
- The following neurological and cardiovascular surgical instrumentswill be down-classified from class III to class II:
- Catheter sterilization connector
- Arterial puncture needle
- Catheter sheath
- Dilator
- Connection valve
- Extension tube
Implementation Requirements for Radio Frequency Therapy Instruments and Radio Frequency Skin Treatment Instruments
The NMPA registration approval must be obtained for radio frequency therapy instruments and radio frequency skin treatment instruments from April 1, 2024, onwards. For already submitted applications but yet to be approved, the NMPA would review and audit according to the previous classification catalogue, however, the registration certificate would also indicate the product’s new classification is the remarks section.
Cisema provides a full range of services to companies seeking NMPA approval or risk classification of their medical device, ensuring the most appropriate route to NMPA approval in China for your product.
Our one-stop service offer for medical device and IVD registration in China, includes:
- Complimentary full-cost calculation including timeline for NMPA registration
- Classification (risk class I, II or III) of medical devices and IVDs
- Clinical pathway strategy
- Templates and document checklists for application
- Writing of product technical requirement (PTR)
- Test sample import and logistics
- Coordination of type tests
- Translation of documents into Chinese
- Chinese label drafting according to NMPA requirements
- Clinical Evaluation Report (CER) composition
- NMPA application dossier compilation
- Responding to supplementary notice and meetings with NMPA reviewers
- Collection and handover of NMPA certificate
- NMPA Legal Agent service for registration and post market surveillance (PMS)
- Clinical Research Organization (CRO) services
- Advice on applicable legislation and relevant regulations
- Post-approval support, e.g. changes, extensions, and renewals of product registrations
To obtain further relevant information, we recommend our exclusive brochures.
By Jacky Li. If you would like to learn more about medical device classification in China or our registration and CRO services for medical devices, IVDs, pharmaceuticals, cosmetics or other products, please contact Cisema.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어