On February 5, 2021, the NMPA (National Medical Products Administration) issued the 2020 Annual Report for Medical Device Registration.
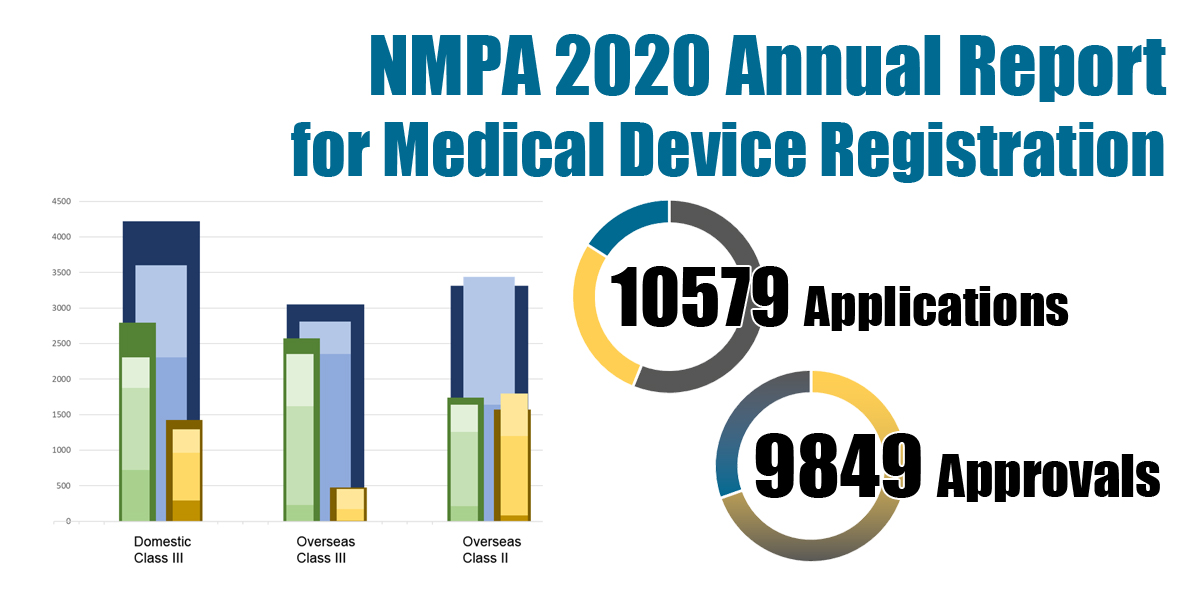
In 2020, the NMPA received a total of 10,579 applications for the initial registration, registration renewals and changes in licensing items of Class III (Domestic and Overseas) and Class II (Overseas) medical devices, increased by 15.6% as compared to 2019. Amongst the 10,579 applications, the NMPA approved a total of 9,849 applications with an increase of 16.3% as compared to 2019. The NMPA handled a total of 1,844 filing applications of imported Class I medical devices, an increase of 33.3% as compared to 2019.
Table 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs

Chart 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs
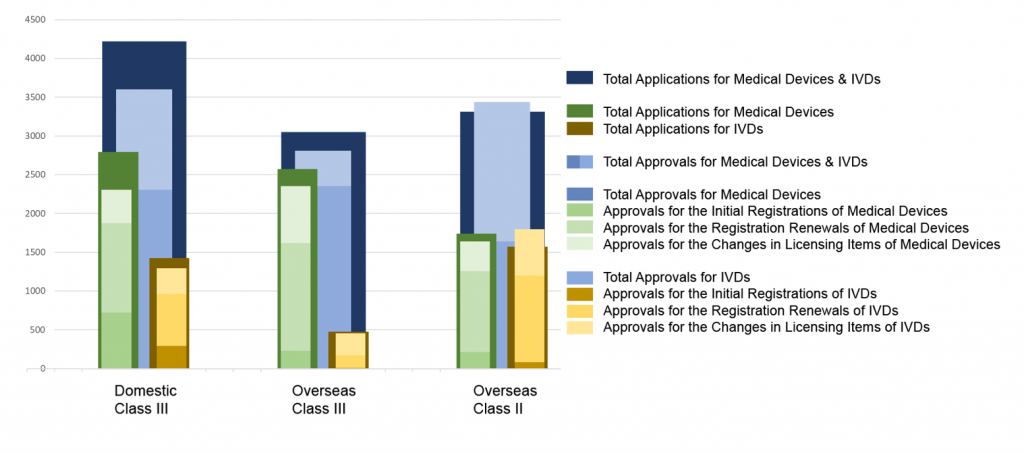
Chart 2. Percentage Distribution of Three Types of NMPA Approvals
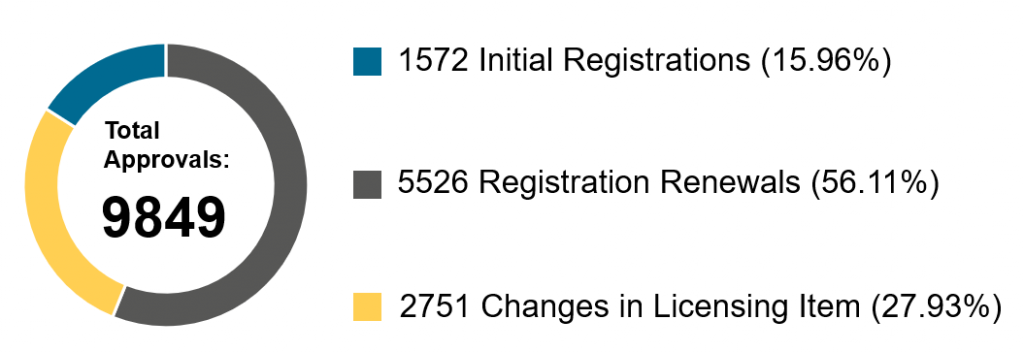
Chart 3. Percentage Distribution of Approvals for Medical Devices and IVDs
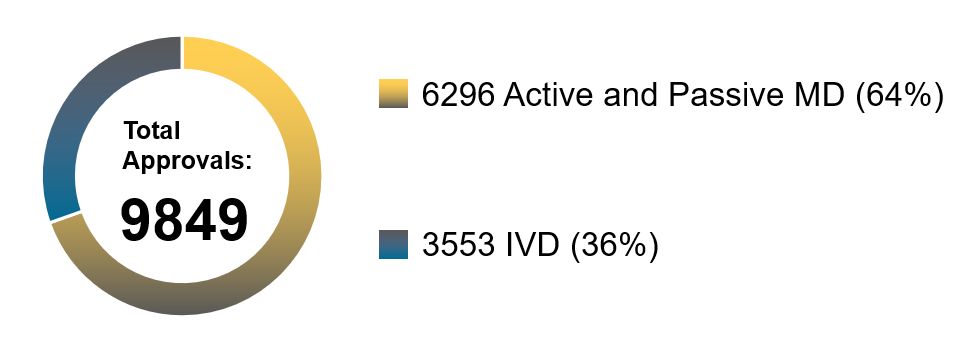
Chart 4. NMPA – No. of Registrations and Applications Approved from January 2015 to December 2020
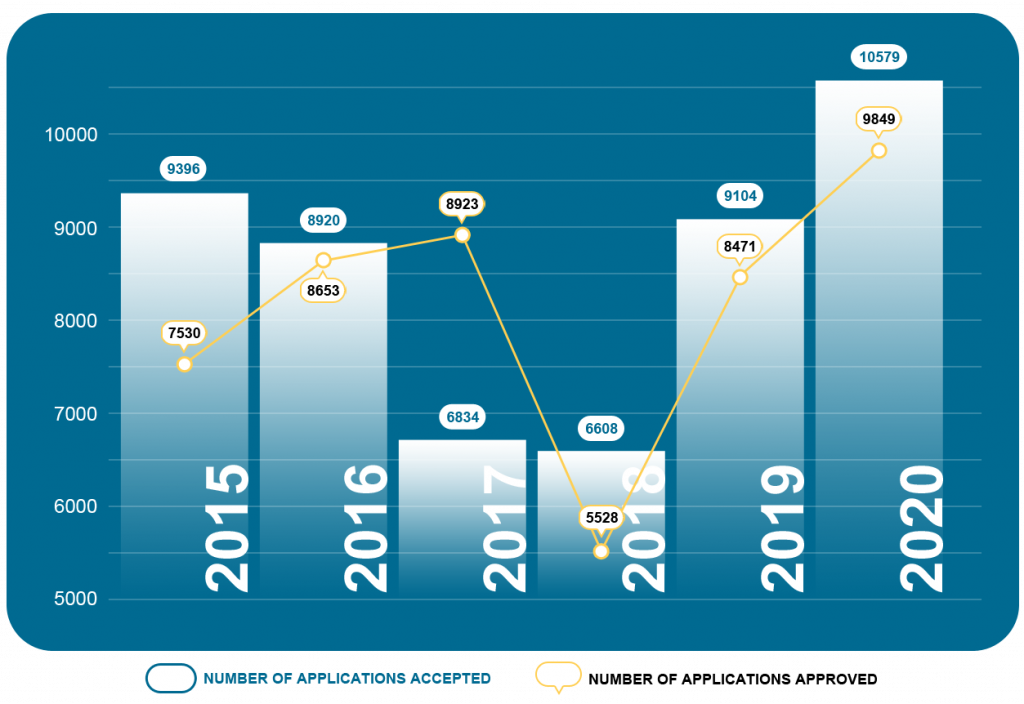
Chart 5. NMPA – No. of New Registrations Approved from January 2020 to December 2020
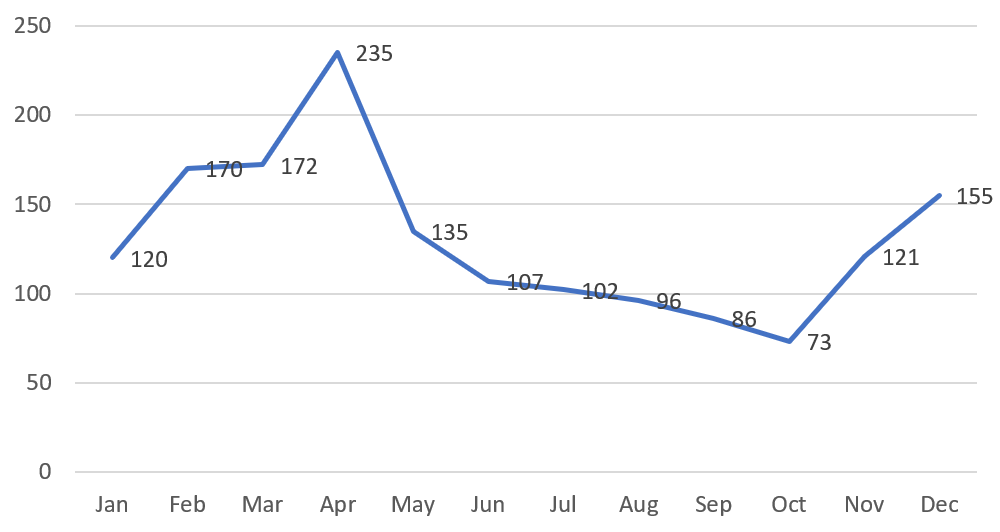
In 2020, the NMPA approved 1,572 new registrations in total.
The provincial medical product administration (MPAs) authorities approved a total of 29,650 applications of China domestic Class II medical devices, an increase of 74.2% as compared to 2019.
The municipal medical product administration (MPAs) authorities handled a total of 34,644 filing applications of China domestic Class I medical devices, an increase of 106.8% as compared to 2019.
The top five class II and III product groups of foreign origin to be registered in 2020 were:
1. Dental Instruments
2. Medical Imaging Equipment
3. Passive Implants Devices
4. Neurological and Cardiovascular Surgical Instruments
5. Ophthalmic Devices
The United States of America, Germany, Japan, Korea and Switzerland continue to have the highest number of initial registrations for overseas medical devices as last year, taking up to 72.3% of the total number of initial registrations for overseas medical devices.
List of Top 10 Countries – Exporting Medical Devices to China
- USA
- Germany
- Japan
- South Korea
- Switzerland
- Taiwan
- France
- Sweden
- Netherland
- Israel
Fast-track Approval for Medical Devices
NMPA received 197 applications for special review and approval of innovative medical devices and 22 applications for priority review. A total of 26 domestic medical devices have obtained the special approval to market as innovative medical devices in 2020.
From 2014 to 2020, the NMPA approved a total of 95 domestic innovative medical devices (78 local brands) and 4 foreign innovative medical devices (4 global brands).
Further information concerning this topic can be obtained from:
Cisema (Hong Kong) Limited
Tel.: +852 3462 2483
info@cisema.com
www.cisema.com/en

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어