On January 27, 2022, the NMPA (National Medical Products Administration) issued the 2021 Annual Report for Medical Device and IVD Registrations.
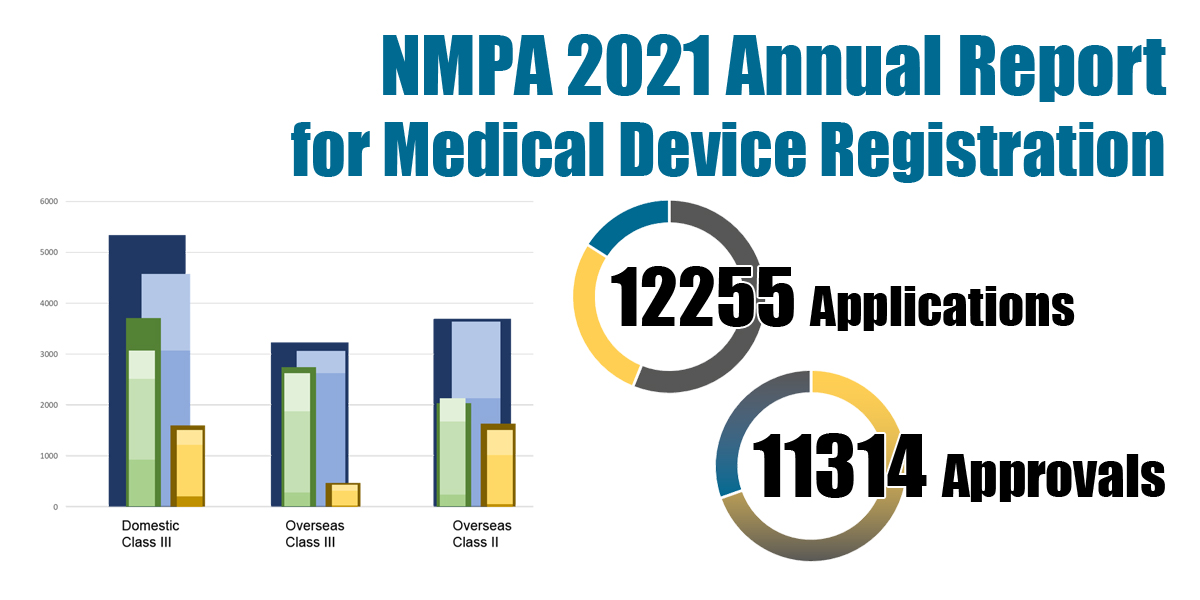
In 2021, the NMPA received a total of 12,255 applications for the initial registration, registration renewals and changes in licensing items of Class III (Domestic and Overseas) and Class II (Overseas) medical devices, increased by 15.8% as compared to 2020. Amongst the 12,255 applications, the NMPA approved a total of 11,314 applications with an increase of 14.9% as compared to 2020. The NMPA handled a total of 1,854 filing applications of imported Class I medical devices, an increase of 0.5% as compared to 2020.
Table 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs

Chart 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs

Chart 2. Percentage Distribution of Three Types of NMPA Approvals

Chart 3. Percentage Distribution of Approvals for Medical Devices and IVDs

Chart 4. NMPA – No. of Registrations and Applications Approved from January 2016 to December 2021

Chart 5. NMPA – No. of New Registrations Approved from January 2021 to December 2021

In 2021, the NMPA approved 1,710 new registrations in total.
The provincial medical product administration (MPAs) authorities approved a total of 31,509 applications of China domestic Class II medical devices, an increase of 6.3% as compared to 2020.
The municipal medical product administration (MPAs) authorities handled a total of 26,773 filing applications of China domestic Class I medical devices, a decrease of 22.7% as compared to 2020.
The top five class II and III product groups of foreign origin to be registered in 2021 were:
1. Medical Imaging Equipment
2. Passive Implants Devices
3. Dental Instruments
4. Neurological and Cardiovascular Surgical Instruments
5. Ophthalmic Devices
The United States of America, Germany, Japan, Korea and Switzerland continue to have the highest number of initial registrations for overseas medical devices as last year, taking up to 72% of the total number of initial registrations for overseas medical devices.
List of Top 10 Countries – Exporting Medical Devices to China
- USA
- Germany
- Japan
- South Korea
- Switzerland
- Italy
- France
- United Kingdom
- Sweden
- Israel
Fast-track Approval for Medical Devices
NMPA received 249 applications for special review and approval of innovative medical devices and 41 applications for priority review. A total of 35 domestic medical devices have obtained the special approval to market as innovative medical devices in 2021.
From 2014 to 2021, the NMPA approved a total of 127 domestic innovative medical devices (104 local brands) and 7 foreign innovative medical devices (5 global brands).
Further information concerning this topic can be obtained from:
Cisema (Hong Kong) Limited
Tel.: +852 3462 2483
info@cisema.com
www.cisema.com/en

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어