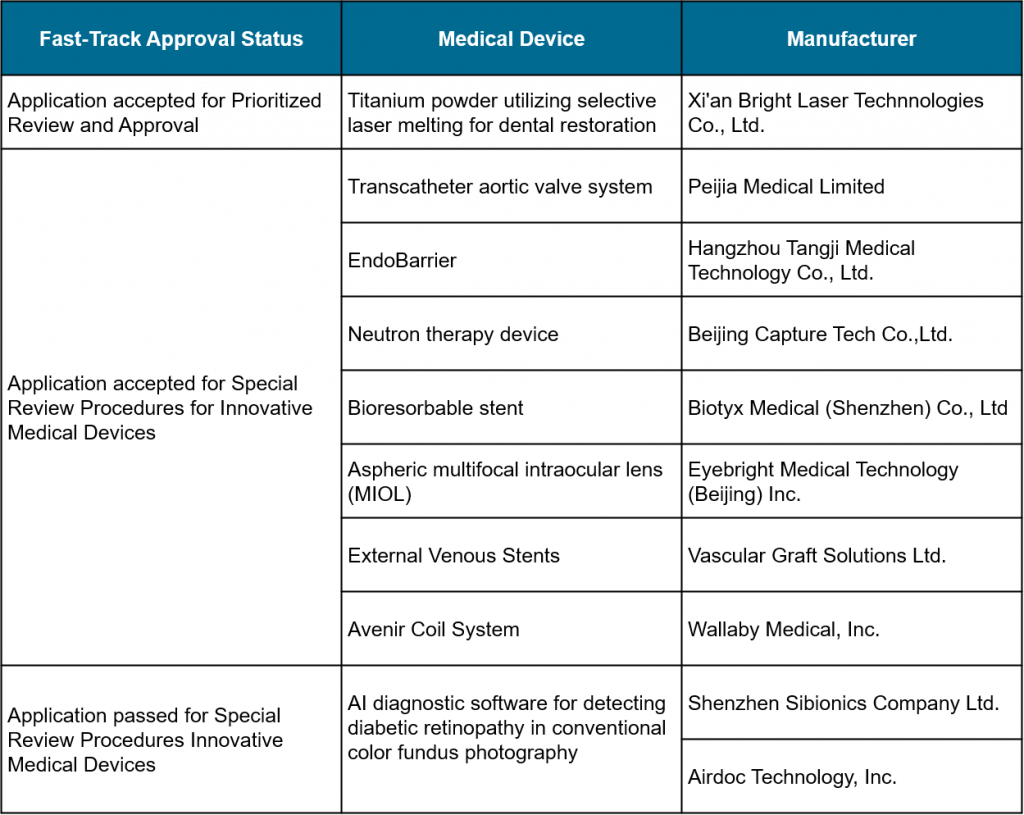
The NMPA (National Medical Products Administration) accepted 7 domestic and 1 overseas applications for fast-track approval and passed 2 other fast-track approvals for the following medical devices.
The “fast-track” for regulatory approval is a policy that was initiated in China from 2014. Whilst there have been no recent regulatory changes to the process, there has been an uptick in approvals as the system matures and more manufacturers seek to take advantage of the regulatory pathway.
AI software has been in the news frequently recently. Read our previous article about the developments for AI medical devices detecting diabetic retinopathy in conventional colour fundus photography.
By Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어