On 10.04.2020, the NMPA (National Medical Products Administration) made the announcement No.25-2020 to provide guidance to NMPA Legal Agents on the timescales for monitoring and reporting medical device adverse events.
A summary of the NMPA Legal Agent responsibilities for monitoring medical device adverse events:
– A well-established monitoring system for medical device adverse events
– Based on the business structure, an appropriate percentage of staffs should be assigned to monitor medical device adverse events
– Collect, report, investigate, analyse and evaluate medical device adverse events in a proactive manner
– Take effective measures for risk control and inform the public in a timely manner
– Continue to conduct research on the safety of already registered medical devices
– Carry out risk assessments and key monitoring work as required and submit relevant reports
– Cooperate with the MPAs in carrying out medical device adverse event monitoring related work
– The NMPA Legal Agent shall establish an information transmission mechanism with overseas manufacturers to promptly exchange information on the adverse event monitoring and re-evaluation of medical devices
– The NMPA Legal Agent shall be registered as an account user of the National Medical Device Adverse Event Monitoring Information System to directly report any adverse events when required
– Responsible for the maintenance of account user and product registration information in a timely manner
– For any changes made to the product registration information, it should be immediately updated in the system
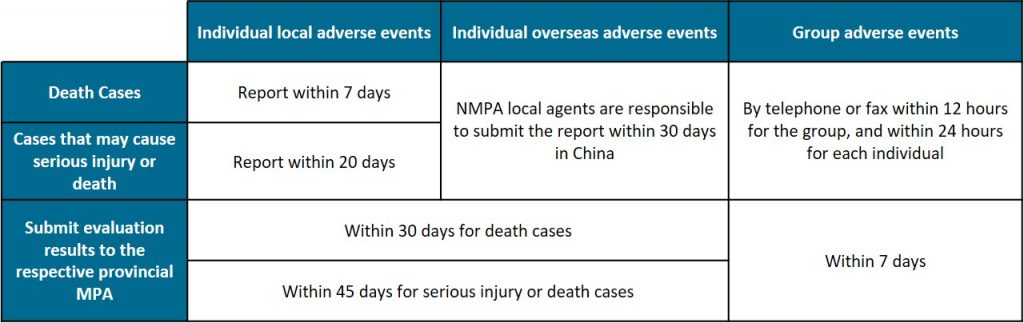
Timescale for adverse events reporting
We note that this announcement should be read together with the NMPA’s new requirements for periodic risk assessment released on 6 May, and further illustrates the NMPA’s increasing emphasis on post-market surveillance.
By Jacky Li. Contact Cisema to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어