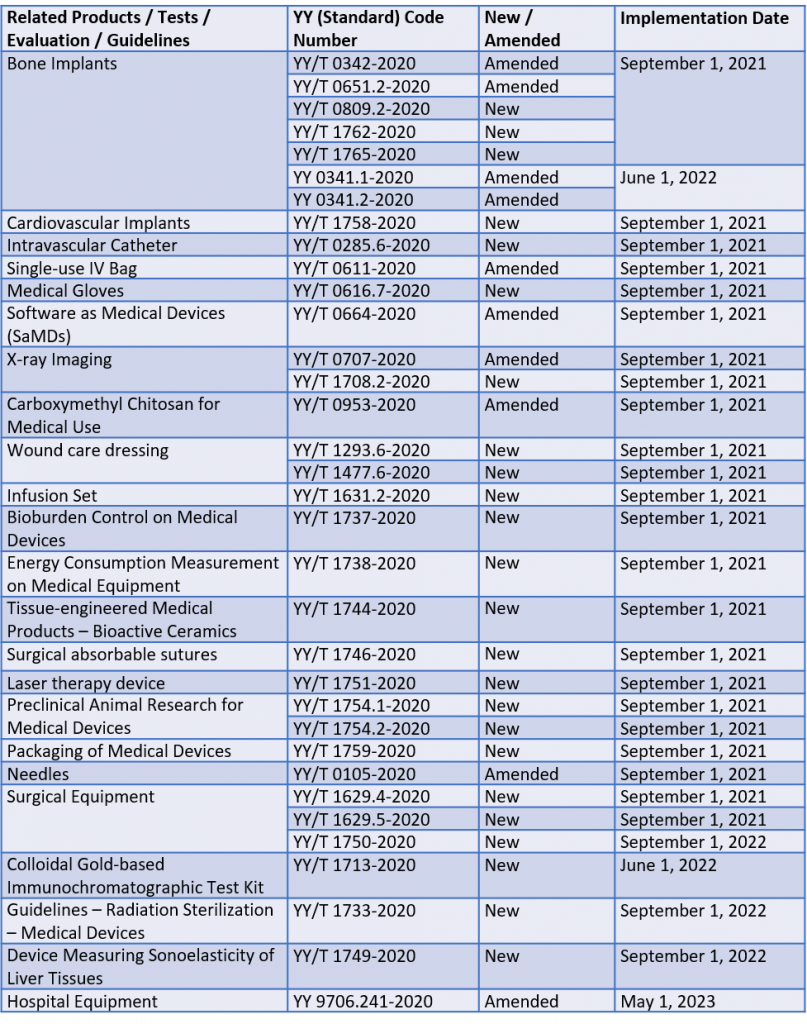
The NMPA (National Medical Products Administration) issued the latest industry requirements, evaluation methods and testing guidelines for bone implants, medical imaging devices, SaMDs (Software as Medical Devices), surgical equipment, test kits, etc. 26 YY standard updates will be implemented first on September 1, 2021. “YY” is the code for mandatory industry standards, while voluntary standards have “/T” added.
The following table prepared by Cisema summarises the key information addressed in the announcement about each YY standard:
By Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어