On 09.04.2020, the Standardization Administration of China newly released GB 9706.1-2020 to replace GB 9706.1-2007 and GB 9706.15-2008 addressing the general requirements for the basic safety and essential performance of the medical electrical equipment. The replacement will take effect in 2023.
The new standard GB 9706.1-2020, equivalent to IEC 60601-1:2012, was announced to be officially implemented on May 1, 2023. The following table shows the transformation from GB 9706.1-1988 to GB 9706.1-2020 and indicates the corresponding IEC standards:
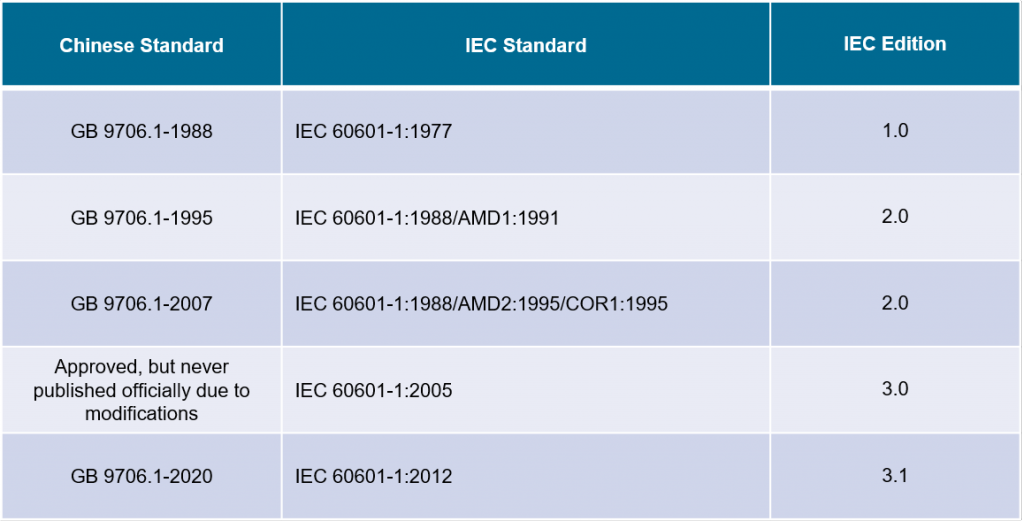
Since 01.07.2008, the GB 9706.1-2007 had been officially implemented as the mandatory standard to ensure the safety of medical electrical equipment available in the China market. In 2014, there was a revision project approved, however, due to the modification of its corresponding standard IEC 60601-1:2005, the new version was never officially published.
IEC 60601-1:2012 had been adopted by overseas manufacturers since 2012, whilst China continued to comply with GB 9706.1-2007. The China GB national standard will be replaced by GB 9706.1-2020 in 2023.
By Jacky Li. Contact Cisema to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어