On December 24, the Center for Medical device Standardization Administration of the NMPA (National Medical Products Administration) issued the second draft of the medical device classification catalogue to solicit public comments. The draft proposed to declassify 7 product categories from class III to class II whilst non-sterile types of liquid and paste dressings will be upclassified as a class II medical device instead of class I.
On December 31, 2021, the NMPA published (No.158 – 2021) the new product catalogue of class I medical devices. The new catalogue covers 19 subdirectories from the previous 2017 version, and consists of 119 primary product categories, 368 secondary product categories, and 2629 product name examples. In addition to the previous version, 90 pieces of product information and 538 product name examples were added. The new catalogue came into effect on January 1, 2022.
For products that have been filed before January 1, 2022, and are still managed as class I medical devices according to the new class I catalogue, if the contents published in the filing information form and the relevant contents of the PTR for filing are inconsistent with the new class I catalogue, the filing applicant shall complete the change of filing information before April 1, 2022. Otherwise, the applicants can propose to the original filing department to cancel the original filing, and re-apply for filing of class I medical devices. IVD test reagents and combination products are not covered in the new catalogue.
Highlights
- Cooling therapeutic devices, light therapy equipment accessories, band-aids, acupressure stimulation apparatus, etc. belonging to the class I risk classification are no longer allowed to contain ingredients such as Chinese medicines, pharmaceutical chemicals, biological products, etc.
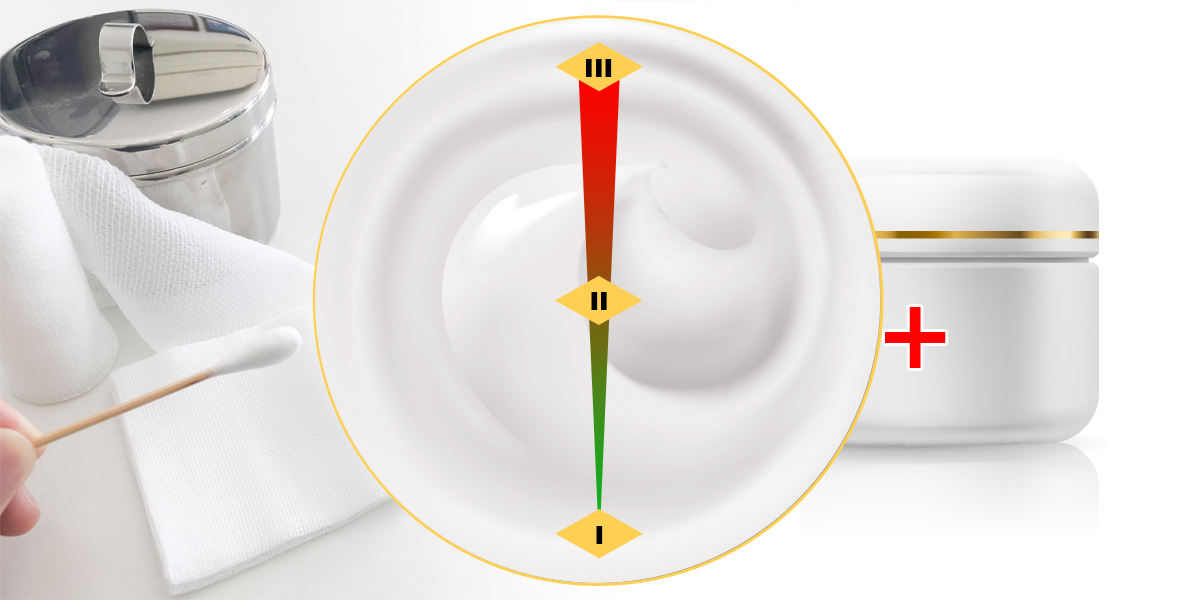
- Previously, liquid and paste dressings can be classified as class I, II and III depending on the intended use, application method, etc., however, non-sterile liquid and paste dressings will no longer be classified as a class I medical device as seen removed from the new class I medical device catalogue. The NMPA’s proposal of the new medical device classification catalogue (draft) aligns with this change.
Medical device classification is the basis for medical device risk management and an important part of the reform of China’s medical device review and approval system. If you want to know more about the classification regulation changes of your products in China, feel free to contact Cisema, and we can help you evaluate.
By Alice Liu and Jacky Li.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어