On October 29, 2021, the NMPA (National Medical Products Administration) published the In Vitro Diagnostic Reagents Classification Rules for the first time as an individual document. Previously, the classification rules for IVDs were only published in the 2014 version of the Administrative Measures for the Registration and Filing of IVDs.
Highlights
Criteria (* for clarified new items) to determine the product classification of:
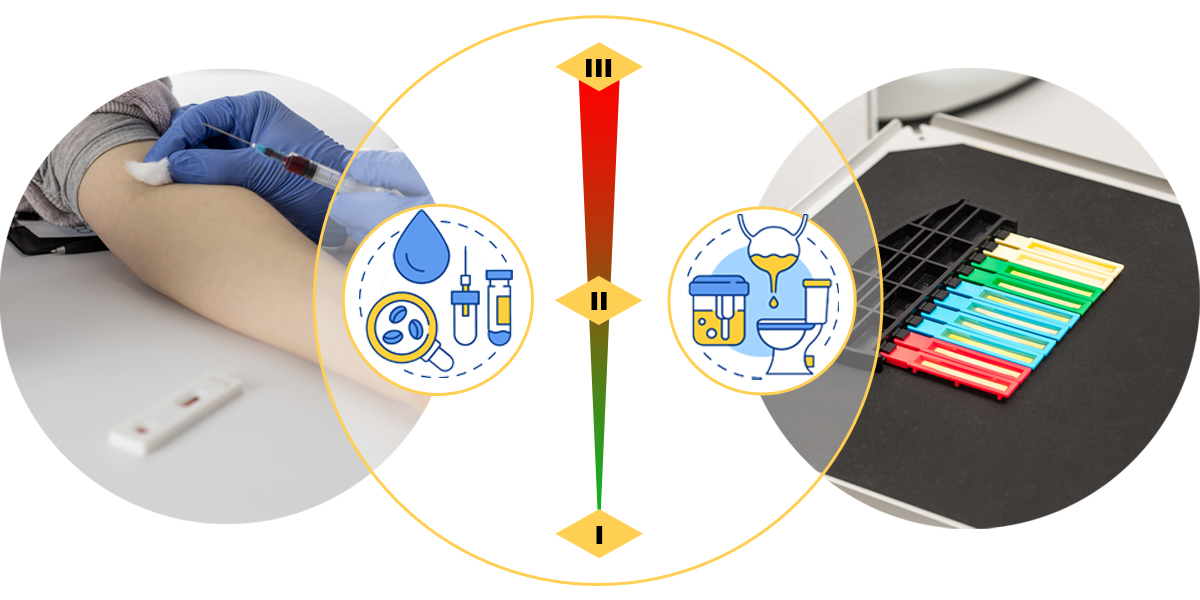
Class I IVD reagents
- Microorganism culture medium (not applicable to microorganism identification and drug sensitivity testing)
- *Intended purpose of Class I IVDs can only be for the proliferation of cell culture (not applicable to cell selection, induction, and differentiation functions)
- Cultured cells can only be intended for the in vitro diagnostics of cell culture medium.
- Products for specimen treatment such as hemolytic agents, diluents, staining solutions, nucleic acid extraction reagent, etc.
- *General reagents for the reaction system such as buffer, substrate solution, enhancement solution, etc.
Class II IVD reagents
- Intended for protein testing.
- *Intended for sugar testing.
- Intended for hormone testing.
- Intended for enzymes testing.
- Intended for esters testing.
- Intended for vitamin testing.
- Intended for inorganic ion testing.
- Intended for the testing of drugs and drug metabolites.
- Intended for auto-antibody testing.
- Intended for microorganism identification and medicine sensitivity experiment.
- *Intended for the proliferation of cell culture (applicable to cell selection, induction, and differentiation functions).
- *Intended for allergen testing (previously classified as Class III).
- Intended for indices inspection such as physiological indices, biochemical indices and immune functions indices.
Class III IVD reagents
- Reagents related to the testing of antigen, antibody and nucleic acid, etc. for pathogen.
- Reagents related to blood type and tissue matching.
- Reagents related to human genetic testing.
- Reagents related to inherited diseases.
- Reagents related to the testing of narcotic drugs, psychotropic drugs and toxic drugs for medical use.
- Reagents related to the testing of targets of curative drugs.
- *Reagents related to the testing for tumor screening, diagnosis, auxiliary diagnosis, staging, etc.
By Alice Liu and Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어