A new policy initiative recently agreed between the Hong Kong and China Mainland governments (read our prior news here) proposes to allow Hong Kong-listed medical devices and drugs to be sold to Hong Kong-owned medical institutions within the Greater Bay Area without prior NMPA registration.
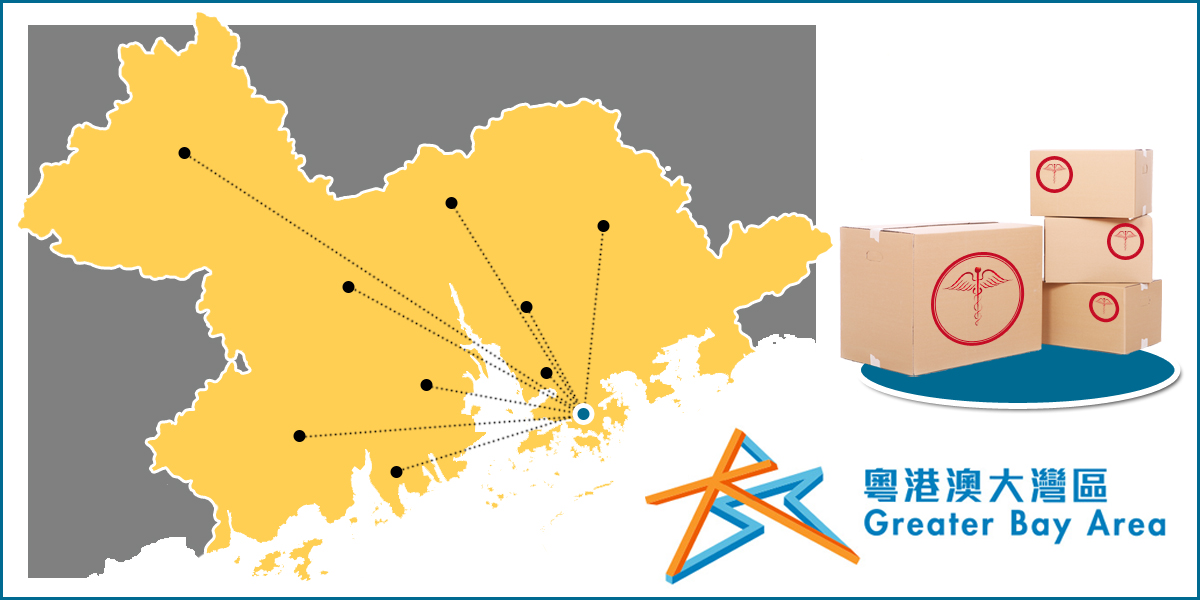
The Chinese Government is pushing to develop the Greater Bay Area, also known as the Pearl River Delta, which includes the cities Guangdong, Hong Kong, Shenzhen and Macau and has a population of 73 million which, to put in perspective, is nearly the size of Germany.
On November 25, 2020, the SAMR (State Administration for Market Regulation) addressed (No. 159 – 2020) the ground breaking action plan for drugs and medical devices in the Greater Bay Area (GBA) as follows:
Temporary measure
- The registration of medical devices in the GBA will be approved and administered by the Guangdong MPA instead of the NMPA
GBA medical institutions eligible to apply
- The GBA medical institutions must be designated by the Guangdong Health Commission for using medical devices imported from Hong Kong.
- The designated medical institutions must be healthcare service providers from Hong Kong or Macao in the form of sole proprietorship, and equity or cooperative joint ventures in the GBA.
- The University of Hong Kong – Shenzhen Hospital would be the first hospital to test-run the medical devices before the policy extends to cover more designated healthcare institutions, drugs and medical devices.
- The China State Council authorizes the Hong Kong Department of Health, as well as Guangdong‘s MPA and Health Commission to administer the approved list of importing medical devices, that are also recognized as:
- Urgently needed for clinical use
- Technological advanced clinical applications
- Procured and already used in Hong Kong (or Macao) public hospitals
Application procedure
- The designated medical institutions in the GBA shall submit the application to the Guangdong MPA
- The Guangdong Health Commission prepares the product evaluation to confirm the following factors:
- The medical device is urgently needed for clinical use
- No other treatments or devices are available for the target patients
- The ability of the medical institution or hospital staff to operate the medical device
- After confirming that there is no similar device registered in China, the Guangdong MPA grants the approval for the medical device to be imported from Hong Kong
The SAMR expects the basic integration of Hong Kong medical devices in GBA medical institutions to be completed by 2022, and a complete supervision system on drug and medical devices to be established by 2035.
At Cisema we are already in communication with the University of Hong Kong – Shenzhen Hospital which is the pilot medical institution for the policy, and can report that they are still finalising the administrative procedures from their side but will be ready to accept applications soon.
By Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어