On 18.03.2020, the NMPA (National Medical Products Administration) issued the 2019 Annual Report for Medical Device Registration.
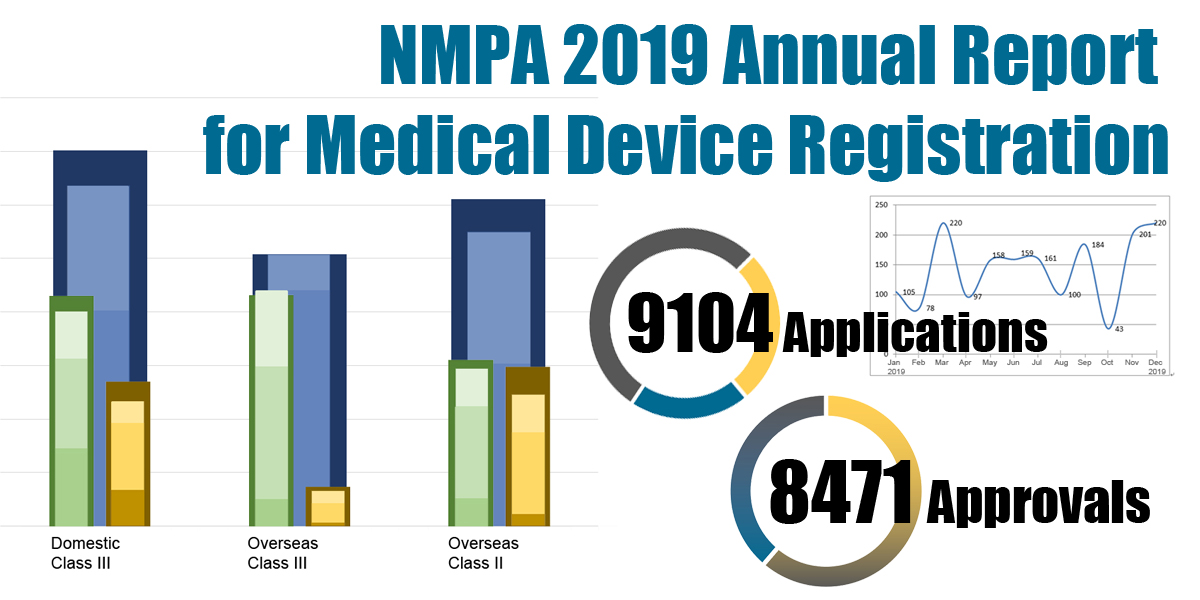
In 2019, the NMPA received a total of 9,104 applications for the initial registration, registration renewals and changes in licensing items of Class III (Domestic and Overseas) and Class II (Overseas) medical devices, an increase of 37.8% from 2018. Amongst the 9,104 applications, the NMPA approved a total of 8,471 applications with an increase of 53.2% as compared to 2018. Also, the NMPA handled a total of 1,383 filing applications of imported Class I medical devices, a decrease of 20.7% as compared to 2018.
Table 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs
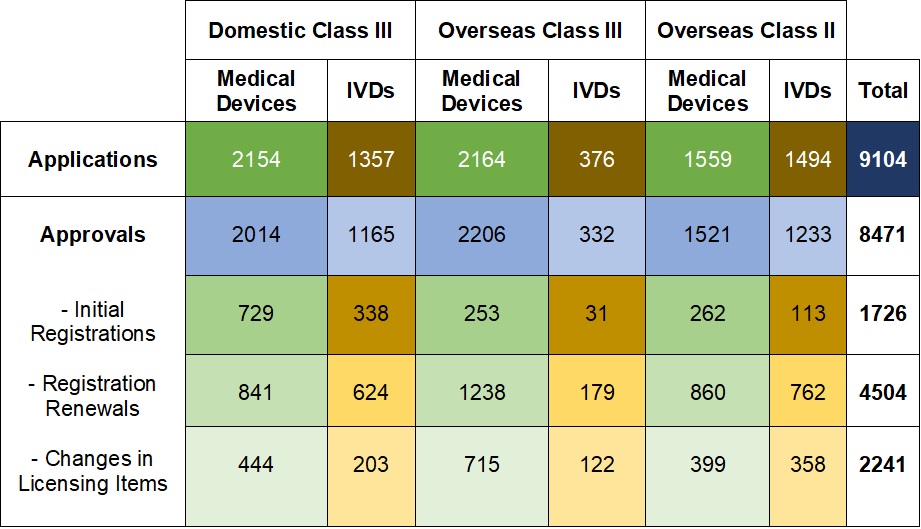
Chart 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs
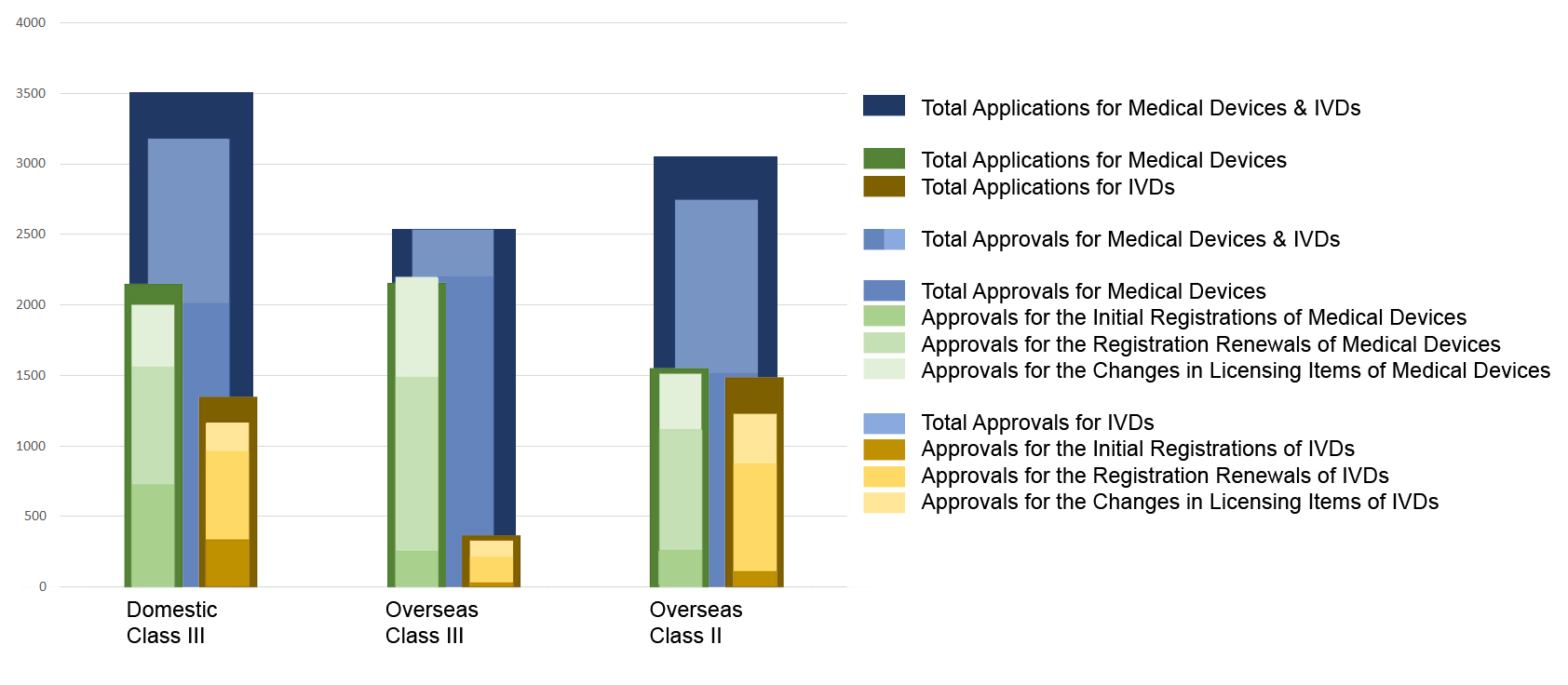
Chart 2. Percentage Distribution of Three Types of NMPA Approvals
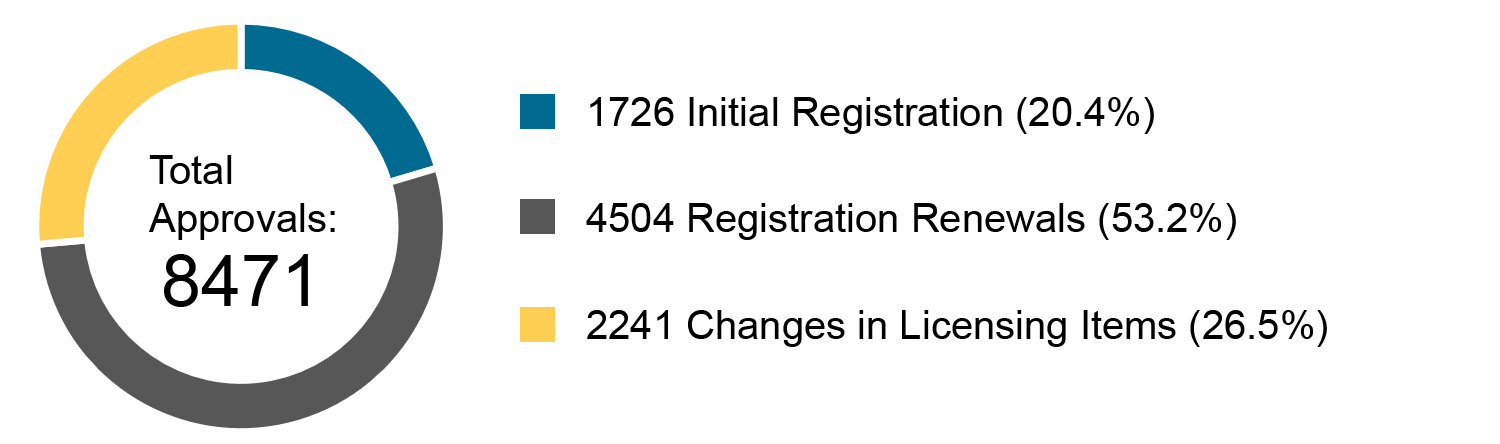
Chart 3. Percentage Distribution of Approvals for Medical Devices and IVDs
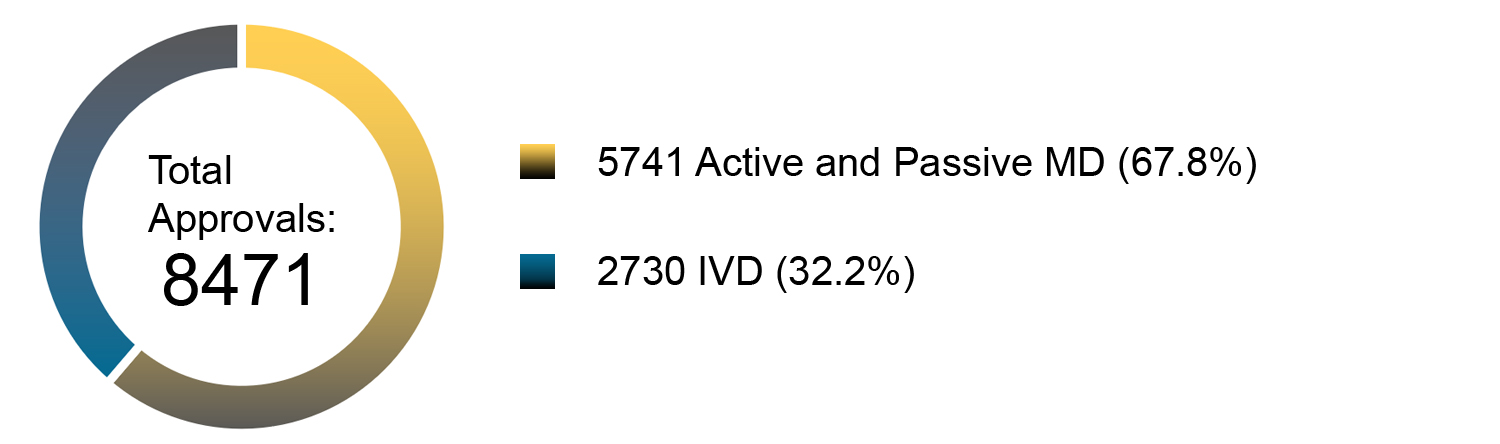
Chart 4. NMPA – No. of New Registrations Approved from January 2013 to December 2019
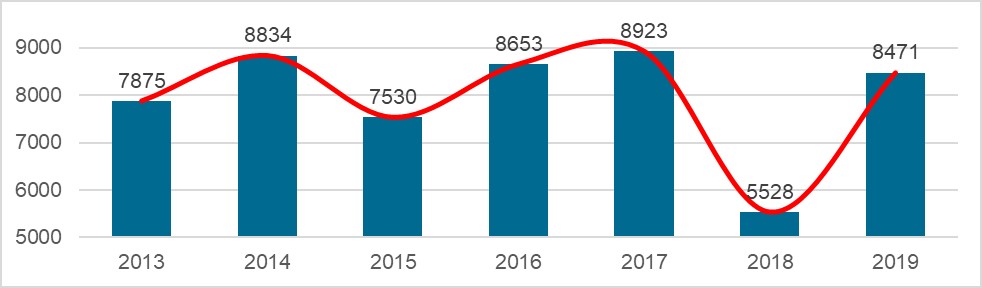
Chart 5. NMPA – No. of New Registrations Approved from January 2019 to December 2019
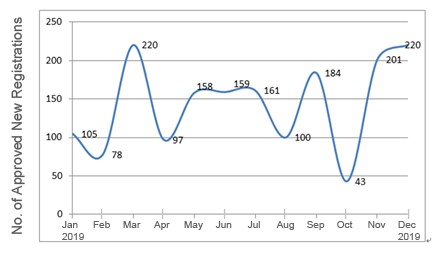
In 2019, the NMPA approved 1,726 new registrations in total.
The provincial medical product administration (MPAs) authorities approved a total of 17,017 applications of China domestic Class II medical devices, an increase of 53.4% from 2018.
The municipal medical product administration (MPAs) authorities handled a total of 16,754 filing applications of China domestic Class I medical devices, decreased by 2.4% as compared to 2018.
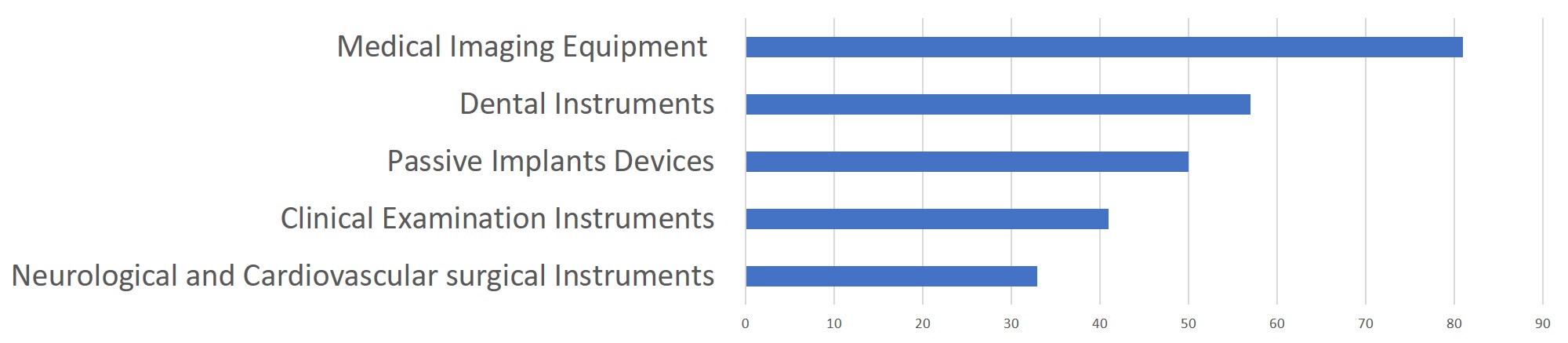
China continues to focus on the supply of high-end, high-cost medical devices from abroad. The top five class II and III product groups of foreign origin to be registered in 2019 were:
1. Medical Imaging Equipment (81 Registrations)
2. Dental Instruments (57 Registrations)
3. Passive Implants Devices (50 Registrations)
4. Clinical Examination Instruments (41 Registrations)
5. Neurological and Cardiovascular Surgical Instruments (33 Registrations)
Chart 6. 2019 Top 5 Product Groups – Registration of Overseas Class II and III Medical Devices
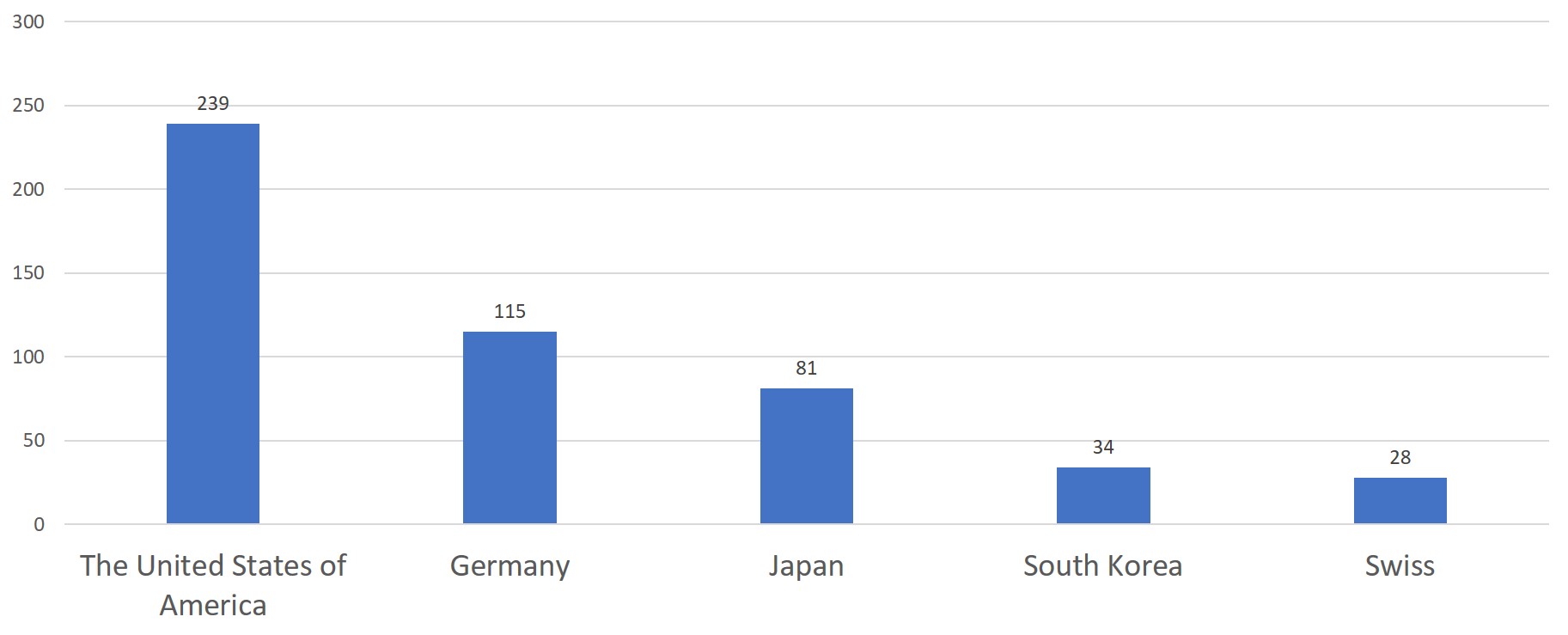
The United States of America, Germany, Japan, Korea and Switzerland have the highest number of initial registrations for overseas medical devices, taking up to 75.4% of the total number of initial registrations for overseas medical devices.
Chart 7. 2019 Top 5 Countries – Exporting Medical Devices to China
Special Review Procedures for Innovative Medical Devices
NMPA received a total of 179 applications for special review and approval of innovative medical devices, including 31 applications for priority review. A total of 19 innovative medical devices were approved:
Imported
1) Micra Transcatheter Leadless Pacemaker system
Domestic
2) PET/CT imaging system
3) Nucleic acid amplification detection analyzer
4) Decellularized corneal implant
5) Left atrial appendage occluder system
6) Abdominal aorta stent-graft and delivery system
7) Bioabsorbable coronary rapamycin-eluting stent system
8) Porous tantalum bone filling material
9) Patient monitor
10) Left atrial appendage closure system
11) Intensity-modulated radiotherapy planning system software
12) Digital Mammography System
13) Transcatheter aortic valve system
14) Single-use intravascular imaging catheter
15) Non-Invasive Blood Glucose Meter
16) Implantable left ventricular assist system
17) Coronary angiographic blood flow reserve fraction measurement system
18) Disposable invasive pressure sensor
19) Positron emission and X-ray computed tomography scanning system
Further information concerning this topic can be obtained from:
Cisema (Hong Kong) Limited
Tel.: +852 3462 2483
info@cisema.com
www.cisema.com/en

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어