On January 26, 2022, the NMPA (National Medical Products Administration) approved (No.12-2022) the supplementary testing methods proposed by the Shandong Institute of Medical Device and Pharmaceutical Packaging Inspection to identify 17 chemical drugs in medical dressing patches.
According to the Shandong Institute of Medical Device and Pharmaceutical Packaging Inspection, manufacturers have illegally added drugs in dressing products in recent years to demonstrate a prominent therapeutic effect. Without carrying out safety evaluation on the product nor indicating the respective drug ingredients in the packaging, consumers are exposed to risks such as skin allergies and skin irritation.
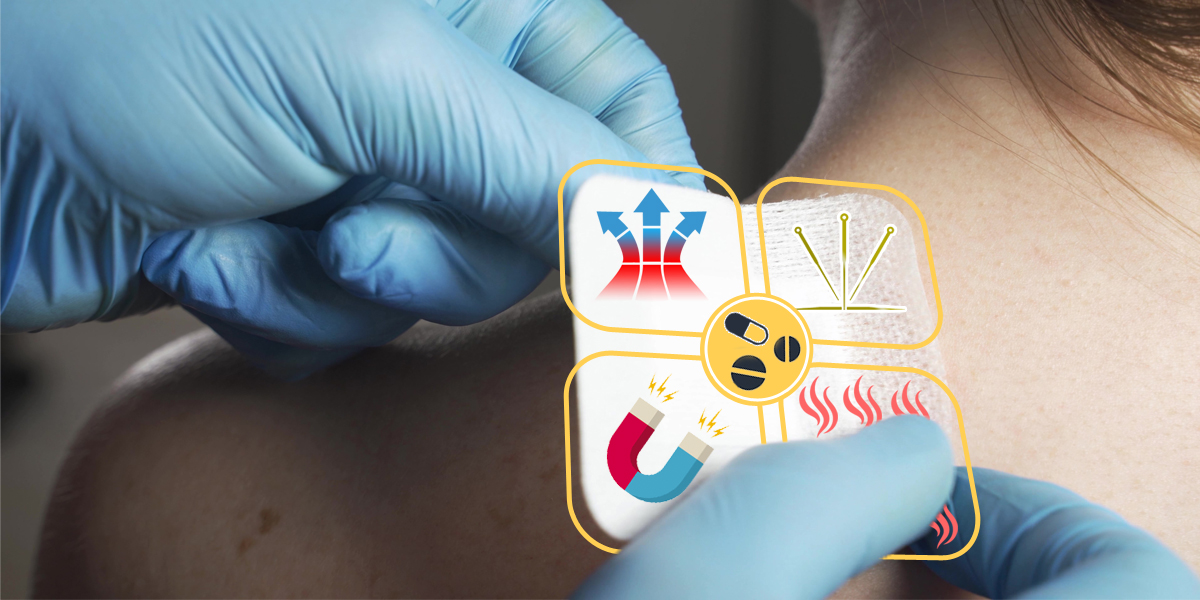
Scope – Medical Dressing Patches
- Only applicable to medical dressing patches with functions of physical warming, physical cooling, acupoint pressure stimulation or utilizing magnetic energy such as far-infrared therapeutic patches, medical cold compress patches, medical antipyretic patches, cold compress gel, magnetotherapy patches, acupuncture point pressing and stimulation stickers, etc.
- Not applicable to thermal therapy stickers and thermal moxibustion stickers that utilize iron powder oxidation and heating as the working principle.
Previously, liquid and paste dressings can be classified as class I, II and III depending on the intended use, application method, etc. However, non-sterile liquid and paste dressings will no longer be classified as a class I medical device as seen removed from the new class I medical device catalogue (No.158 – 2021) which came into effect on January 1, 2022. (More information available in our previous blog post.)
By Julie Zhang and Jacky Li. Contact Cisema if you would like to learn more.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어