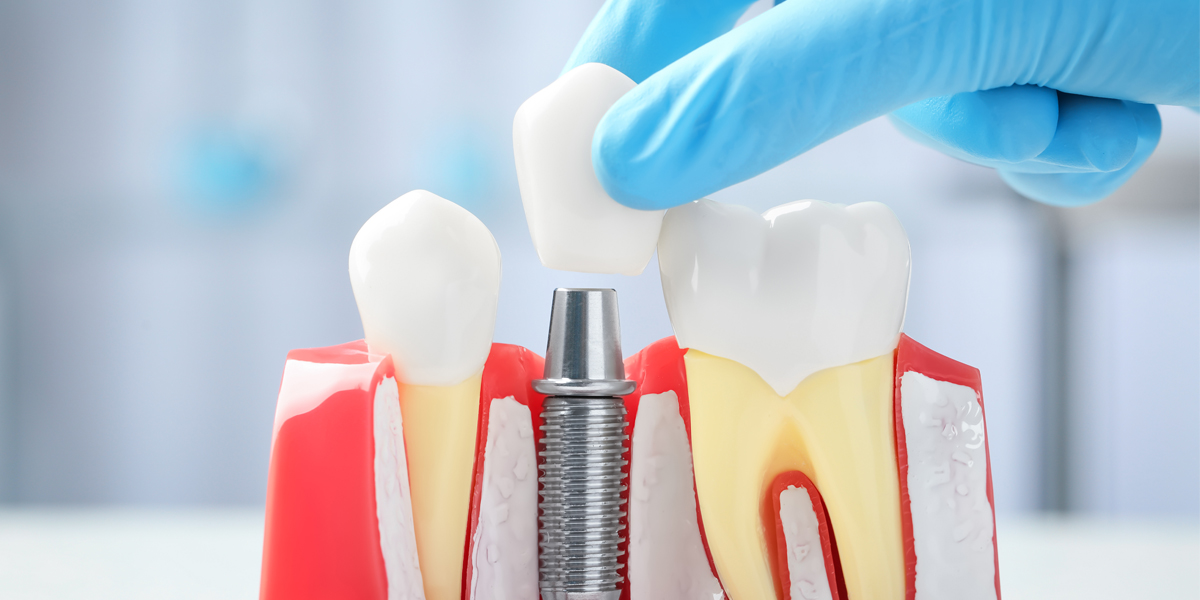
Clinical evaluation of dental implant system has new guidelines (No.23-2023) issued by the China Center Medial Device of Evaluation (CMDE) on August 14, 2023. The purpose of the guidance document is to establish a standardized framework for evaluating the clinical performance of dental implant systems and outline the principles for conducting clinical evaluations within the same category of dental implant systems.
Scope
This guidelines primarily apply to dental implant systems retained within the oral cavity after surgical procedures, excluding custom implant systems. The clinical evaluation involves comparing the applicant’s product with other similar products using clinical data. The scope covers products that are considered class III in China and classified under the code 17-08-01 in the medical device catalogue.
Comparative analysis
When conducting clinical evaluations through comparative analysis of products of the same type, applicants have the flexibility to choose one or more similar products for comparison. Priority should be given to products with similar scope of application and technological characteristics. In the comparative analysis, the guidelines advise a focus on material, structural design, surface modification methods, and key technological features.
Comparison of Clinical Application and Usage Information
The comparison of clinical application and usage information should focus on specific aspects such as the application sites, clinical methods, usage procedures, compatibility, and contraindications of the applicant’s product and the reference product.
Comparison of Technical Characteristics
A detailed comparison of technical aspects including design, materials, product performance, and other crucial technical features should be undertaken. Elements like product structure, material properties, and compatibility with accessories should be analyzed.
Safety and Efficacy Assessment for Differences
In cases where differences between the applicant’s product and reference products are identified, evidence should be provided to demonstrate that these differences do not adversely affect safety and efficacy. This may include comparative data, real-world measurements, and animal studies. These data collectively build a case for the safety and effectiveness of the product under review.
Clinical Data Evaluation
Applicants are required to submit clinical data for both the applicant’s product and reference products. These data should be comprehensive and include information about the implant type, treatment type, follow-up periods, success rates, complications, and adverse events.
Further information
The principles outlined in this guidance document serve as a valuable resource for regulatory evaluation in the field of dental implant systems. They provide clear instructions for both applicants and regulators to ensure that dental implant products meet the necessary standards of safety and efficacy. Read the original article on clinical evaluation registration of dental implant system with the same species.
As technology and regulations evolve, the guidance will be updated to align with the latest advancements in the field. By adhering to the outlined requirements, manufacturers from the dental implant industry can ensure their products meet the necessary safety and performance standards. Also, Cisema can assist manufacturers in navigating the registration process effectively. Discover our services for medical device registration, renewals and NMPA Legal Agent.

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어