China’s giant e-commerce platform provider JD.com recently reported that JD’s online sales of disinfectants in China increased by more than eight times in February during the peak of the COVID-19 pandemic crisis in comparison to the same period of last year.[1]Other e-commerce platforms have reported similar surges in sales.[2][3] Due to the increased demand, China may be perceived as a very attractive market for overseas disinfectant manufacturers, but before their products can be imported and sold, they must comply with the local regulatory framework.
In China, disinfectants are defined as “preparations that are used to kill microorganisms on the transmission medium to achieve disinfection or sterilization requirements”.[4]
In China, disinfectants are further classified into risk classes depending on their ingredients and intended use. Products of Class I and II are those that have the highest risk to users, whereas products of Class III have the lowest risk.[5]
Because of the low risk of Class III to users, products of this class do not have to be approved by the relevant authorities to be sold in China. But all disinfectants of Class I and II must be filed with the provincial health authorities, e.g. Beijing Municipal Health Commission.
Prior to being eligible to file, a product must have obtained a testing report by an accredited Chinese laboratory to evaluate the product claims. The stability test, which determines what amount of the active ingredient is lost over time, can be quite lengthy because it depends on the product’s shelf life. Accelerated stability tests can be conducted at higher temperatures to shorten the testing window and accelerate time to market.
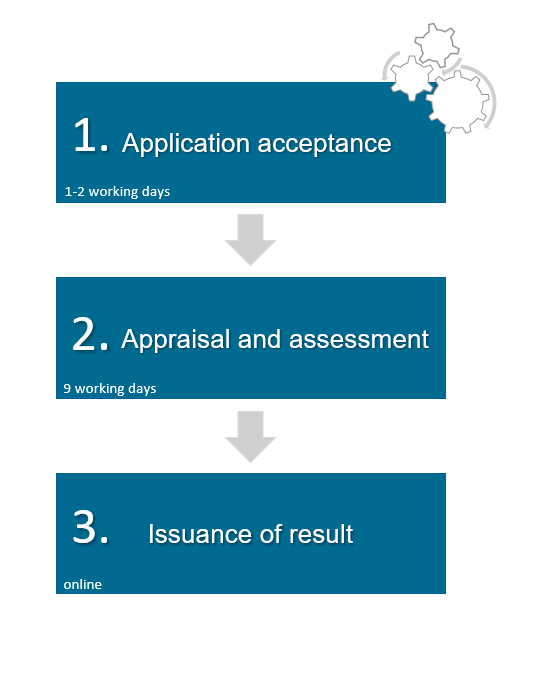
Once a test certificate is obtained, the filing process is relatively quick and straightforward as follows:
(1) acceptance of the application (1-2 working days);
(2) appraisal and assessment (9 working days); and
(3) online issue of the registration certificate as soon as the positive assessment result is available.
Once the positive assessment result is available, the listing will be posted online and can be accessed through the national online information service platform for disinfections products (https://credit.jdzx.net.cn/xdcp/loginPage.do). No physical certificate is issued.
Class II filings do not have an expiration date, however producers of Class I disinfectants, which are the highest risk, must renew their filing every 4 years.
The filing process for approval of disinfectants of Class I and II in China
The filing process itself usually takes no more than 20 days. However, when considering the time for preparation and testing (e.g. stability test, safety test, etc.) the whole process may take six months or longer. The application process for New-DP is more complex and may take even longer because of the required approval and examination of the disinfectant’s new component (e.g. new ingredient) by the National Health Commission, which must be conducted before the actual filing process. Once the New-DP is approved, it will thereafter be treated as a NN-DP.
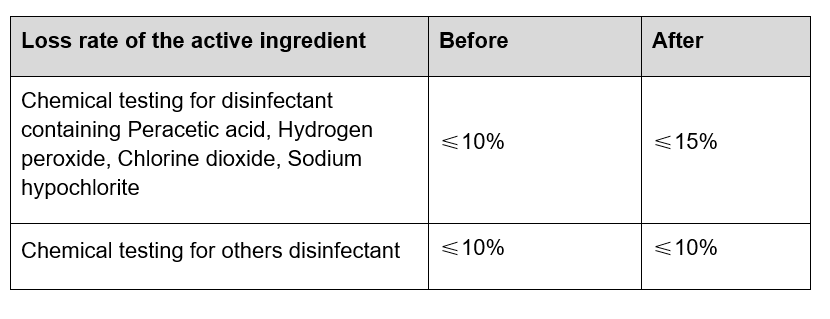
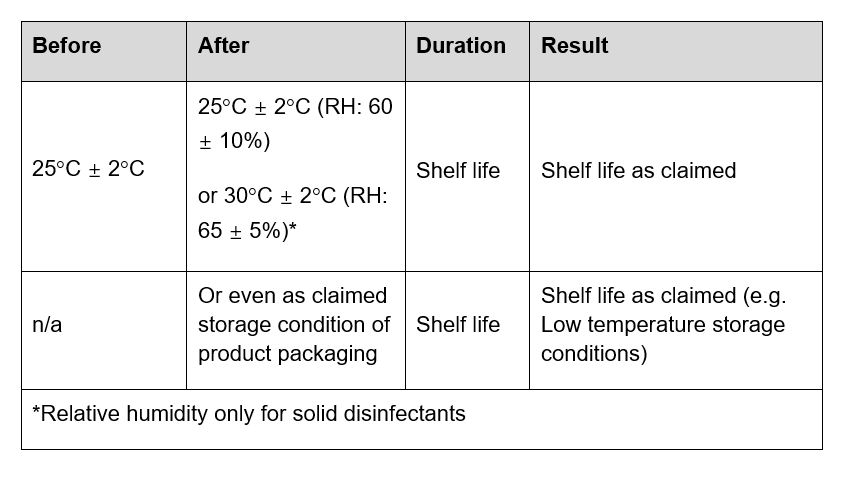
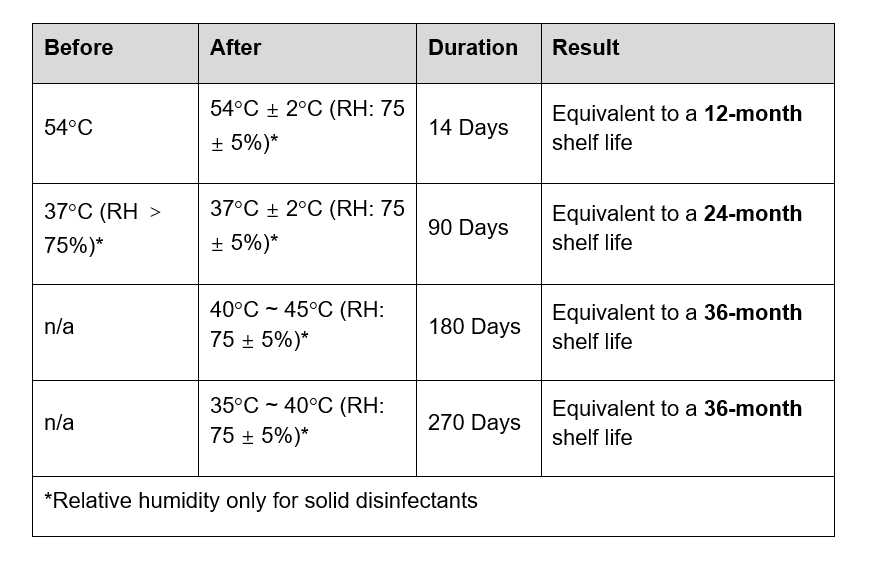
On 1st October 2020, the new GB/T 38499-2020: Evaluation method for stability of disinfectant will come into effect, which updates the regulations and conditions for stability tests of disinfectants. In particular, disinfectants may undergo stability tests on the basis of the product claim (such as where a product requires storage at lower temperatures), rather than on the basis of standardised measures.
A table setting out the old and new requirements follows below.
Table: New GB/T 38499-2020
Evaluation requirements for stability
Long term storage test
Accelerated storage test for stability
By Anneli Nguyen. Contact Cisema if you want to learn more or for a full-cost calculation to register your disinfectant for sale in China.
[1] Yuchuan Wang, “JD sales data shows strong trend of returning to work” (03.03.20)
https://jdcorporateblog.com/jd-sales-data-shows-strong-trend-of-returning-to-work/
Last access on 07.07.20
[2] Sarah Dai, “China’s e-commerce players look to ease coronavirus fears by freezing prices for masks, disinfectant” (22.01.20)
https://www.scmp.com/tech/policy/article/3047117/chinas-e-commerce-players-look-ease-coronavirus-fears-freezing-prices
Last access on 07.07.20
[3] Justin Harper, “Coronavirus: Taobao warns firms not to profit from outbreak” (23.01.20)
https://www.bbc.com/news/business-51202519
Last access on 07.07.20
[4] Section 1.3.5 of “Technical Standard for disinfection (2002 edition)”, which was drafted by the Ministry of Health of the People’s Republic of China in 2002; the same description can be found in chapter 3.1 of GB 38850-2020 (coming into effect on 2020-11-01).
[5] Same as above footnote 4

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어 



