Medical device registrations China 2022 Annual Report was issued by the NMPA (National Medical Products Administration) on February 8, 2023.
In 2022, the NMPA received a total of 10,571 applications for the initial registration, registration renewals and changes in licensing items of Class III (Domestic and Overseas) and Class II (Overseas) medical devices, decreased by 13.7% as compared to 2021. The NMPA approved a total of 11,942 applications with an increase of 5.5% as compared to 2021. The NMPA handled a total of 2,023 filing applications of imported Class I medical devices, an increase of 9.1% as compared to 2021.
Table 1. NMPA – No. of Applications and Approvals for Medical Devices and IVDs
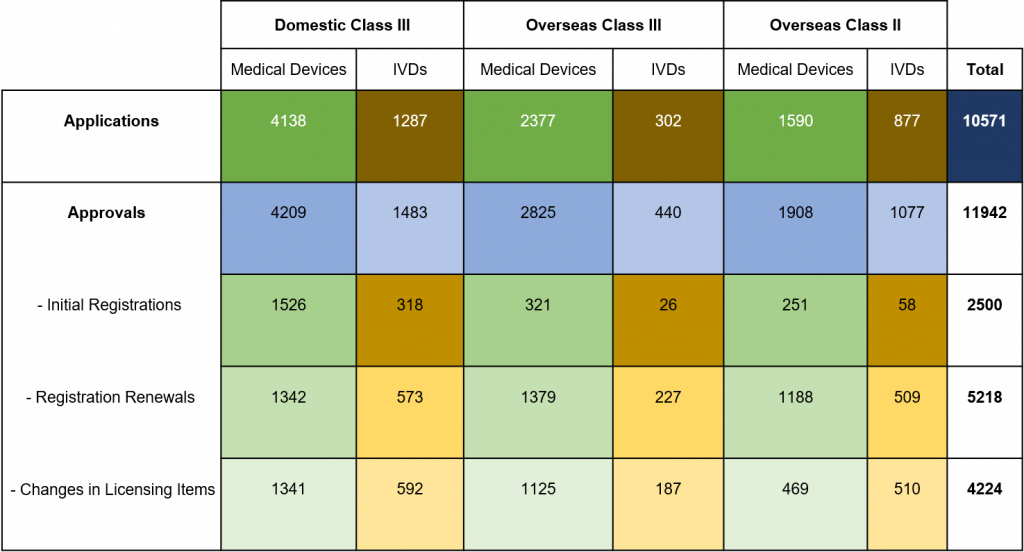
Chart 2. Percentage Distribution of Three Types of NMPA Approvals

Chart 3. Percentage Distribution of Approvals for Medical Devices and IVDs
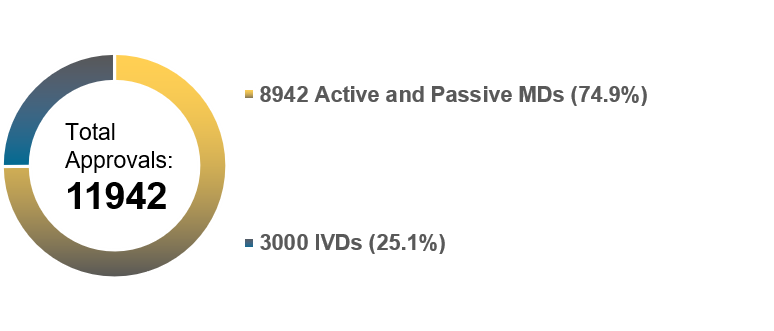
Chart 4. NMPA – No. of Registrations and Applications Approved from January 2016 to December 2022
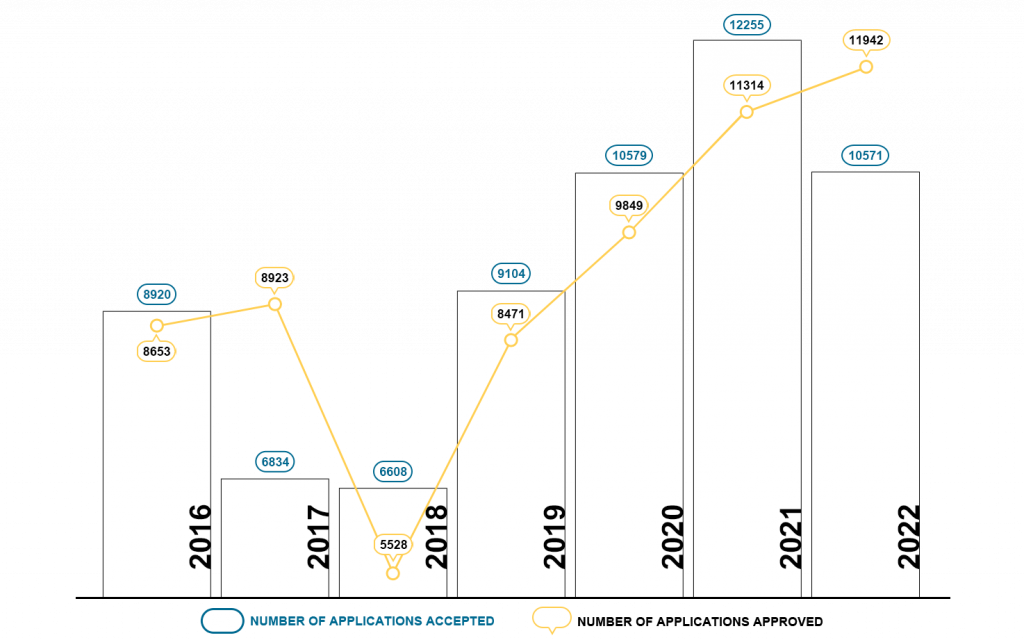
Chart 5. NMPA – No. of New Registrations Approved from January 2022 to December 2022
In 2022, the NMPA approved 2,500 new registrations in total.
The provincial medical product administration (MPAs) authorities approved a total of 32,889 applications of China domestic Class II medical devices, an increase of 4.4% as compared to 2021.
The municipal medical product administration (MPAs) authorities handled a total of 28,508 filing applications of China domestic Class I medical devices, an increase of 6.5% as compared to 2021.
The top five class II and III product groups of foreign origin to be registered in 2022 were:
1. Medical imaging devices
2. Passive implant devices
3. Infusion, nursing and protective equipment
4. Dental devices
5. Neurological and cardiovascular surgical devices
The United States of America, Germany, Japan, and Korea continue to have the highest number of initial registrations for overseas medical devices as last year, taking up to 72% of the total number of initial registrations for overseas medical devices.
List of Top 10 Countries – Exporting Medical Devices to China
- USA
- Germany
- Japan
- South Korea
- France
- Switzerland
- United Kingdom
- Italy
- Sweden
- Israel
Fast-track Approval for Medical Devices
NMPA received 343 applications for special review and approval of innovative medical devices. A total of 55 medical devices have obtained the special approval to market as innovative medical devices in 2021.
From 2014 to 2022, the NMPA approved a total of 189 innovative medical devices manufactured by 134 domestic and 8 overseas enterprises.
Further information
Refer to NMPA’s original medical device registrations China 2022 annual report.
Further information concerning this topic can be obtained from contacting us:
Cisema (Hong Kong) Limited
Tel.: +852 3462 2483
officehk@cisema.com
www.cisema.com/en

 Deutsch
Deutsch  Italiano
Italiano  Français
Français  日本語
日本語  한국어
한국어 



